
Microglia are a type of neuroglia located throughout the brain and spinal cord. Microglia account for about 10-15% of cells found within the brain. As the resident macrophage cells, they act as the first and main form of active immune defense in the central nervous system (CNS). Microglia originate in the yolk sac under a tightly regulated molecular process. These cells are distributed in large non-overlapping regions throughout the CNS. Microglia are key cells in overall brain maintenance—they are constantly scavenging the CNS for plaques, damaged or unnecessary neurons and synapses, and infectious agents. Since these processes must be efficient to prevent potentially fatal damage, microglia are extremely sensitive to even small pathological changes in the CNS. This sensitivity is achieved in part by the presence of unique potassium channels that respond to even small changes in extracellular potassium. Recent evidence shows that microglia are also key players in the sustainment of normal brain functions under healthy conditions. Microglia also constantly monitor neuronal functions through direct somatic contacts and exert neuroprotective effects when needed.

Alpidem, sold under the brand name Ananxyl, is a nonbenzodiazepine anxiolytic medication which was briefly used to treat anxiety disorders but is no longer marketed. It was previously marketed in France, but was discontinued due to liver toxicity. Alpidem is taken by mouth.

PK-11195 is an isoquinoline carboxamide which binds selectively to the peripheral benzodiazepine receptor (PBR). It is one of the most commonly used PBR ligands due to its high affinity for the PBR in all species, although it is starting to be replaced by newer and more selective ligands.

Translocator protein (TSPO) is an 18 kDa protein mainly found on the outer mitochondrial membrane. It was first described as peripheral benzodiazepine receptor (PBR), a secondary binding site for diazepam, but subsequent research has found the receptor to be expressed throughout the body and brain. In humans, the translocator protein is encoded by the TSPO gene. It belongs to a family of tryptophan-rich sensory proteins. Regarding intramitochondrial cholesterol transport, TSPO has been proposed to interact with StAR to transport cholesterol into mitochondria, though evidence is mixed.

Kv7.2 (KvLQT2) is a voltage- and lipid-gated potassium channel protein coded for by the gene KCNQ2.

Etifoxine, sold under the trade name Stresam among others, is a nonbenzodiazepine anxiolytic agent, primarily indicated for short-term management of adjustment disorder, specifically instances of situational depression accompanied by anxiety, such as stress-induced anxiety. Administration is by mouth. Side effects associated with etifoxine use include slight drowsiness, headache, skin eruptions, and allergic reactions. In rare cases, etifoxine has been linked to severe skin and liver toxicity, as well as menstrual bleeding between periods. Unlike benzodiazepines, etifoxine does not cause sedation or lack of coordination. Etifoxine acts as a GABAA receptor positive allosteric modulator and as a ligand for translocator proteins. Both mechanisms are conjectured to contribute to its anxiolytic properties.
A cannabinoid receptor antagonist, also known simply as a cannabinoid antagonist or as an anticannabinoid, is a type of cannabinoidergic drug that binds to cannabinoid receptors (CBR) and prevents their activation by endocannabinoids. They include antagonists, inverse agonists, and antibodies of CBRs. The discovery of the endocannabinoid system led to the development of CB1 receptor antagonists. The first CBR inverse agonist, rimonabant, was described in 1994. Rimonabant blocks the CB1 receptor selectively and has been shown to decrease food intake and regulate body-weight gain. The prevalence of obesity worldwide is increasing dramatically and has a great impact on public health. The lack of efficient and well-tolerated drugs to cure obesity has led to an increased interest in research and development of CBR antagonists. Cannabidiol (CBD), a naturally occurring cannabinoid and a non-competitive CB1/CB2 receptor antagonist, as well as Δ9-tetrahydrocannabivarin (THCV), a naturally occurring cannabinoid, modulate the effects of THC via direct blockade of cannabinoid CB1 receptors, thus behaving like first-generation CB1 receptor inverse agonists, such as rimonabant. CBD is a very low-affinity CB1 ligand, that can nevertheless affect CB1 receptor activity in vivo in an indirect manner, while THCV is a high-affinity CB1 receptor ligand and potent antagonist in vitro and yet only occasionally produces effects in vivo resulting from CB1 receptor antagonism. THCV has also high affinity for CB2 receptors and signals as a partial agonist, differing from both CBD and rimonabant.

AM-694 (1-(5-fluoropentyl)-3-(2-iodobenzoyl)indole) is a designer drug that acts as a potent and selective agonist for the cannabinoid receptor CB1. It is used in scientific research for mapping the distribution of CB1 receptors.

Emapunil is an anxiolytic drug which acts as a selective agonist at the peripheral benzodiazepine receptor, also known as the mitochondrial 18 kDa translocator protein or TSPO. This protein has multiple functions, among which is regulation of steroidogenesis, particularly the production of neuroactive steroids such as allopregnanolone in the brain. In both animal and human trials, emapunil produced fast acting anxiolytic and anti-panic effects, without producing sedation or withdrawal symptoms following cessation of use. Emapunil is also used in its 11C radiolabelled form to map the distribution of TSPO receptors in the brain.
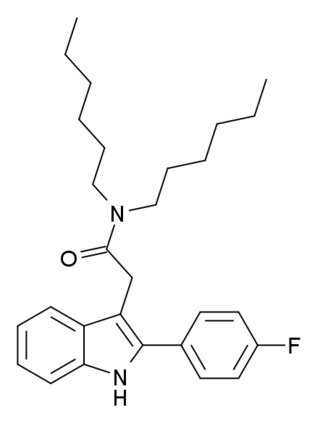
FGIN-1-27 is an anxiolytic drug which acts as a selective agonist at the peripheral benzodiazepine receptor, also known as the mitochondrial 18 kDa translocator protein or TSPO. It is thought to produce anxiolytic effects by stimulating steroidogenesis of neuroactive steroids such as allopregnanolone.

FGIN-1-43 is an anxiolytic drug which acts as a selective agonist at the peripheral benzodiazepine receptor, also known as the mitochondrial 18 kDa translocator protein or TSPO. It is thought to produce anxiolytic effects by stimulating steroidogenesis of neuroactive steroids such as allopregnanolone, and is several times more potent than the related drug FGIN-127.

SSR-180,575 is a drug which acts as a selective agonist at the peripheral benzodiazepine receptor, also known as the mitochondrial 18 kDa translocator protein or TSPO. It has been shown to have neuroprotective and cardioprotective effects and to stimulate steroidogenesis of pregnenolone in the brain, which may be linked to its neuroprotective action.

DAA-1097 is a drug which acts as a potent and selective agonist at the peripheral benzodiazepine receptor, also known as the mitochondrial 18 kDa translocator protein or TSPO, but with no affinity at central benzodiazepine receptors. It has anxiolytic effects in animal studies.
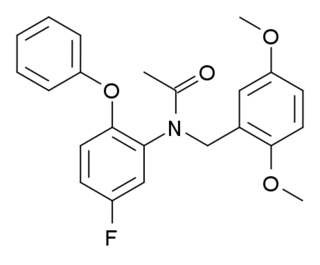
DAA-1106 is a drug which acts as a potent and selective agonist at the peripheral benzodiazepine receptor, also known as the mitochondrial 18 kDa translocator protein or TSPO, but with no affinity at the GABAA receptor. It has anxiolytic effects in animal studies. DAA-1106 has a sub-nanomolar binding affinity (Ki) of 0.28nM, and has been used extensively in its 3H or 11C radiolabelled form to map TSPO in the body and brain, which has proved especially helpful in monitoring the progress of neurodegenerative diseases such as Alzheimer's disease.

Ro5-4864 (4'-chlorodiazepam) is a drug which is a benzodiazepine derivative of diazepam. However unlike most benzodiazepine derivatives, Ro5-4864 lacks affinity for GABAA receptors and lacks typical benzodiazepine effects, instead being sedative yet also convulsant and anxiogenic in effects. Ro5-4864 was found to be a potent ligand for the "peripheral benzodiazepine receptor", later renamed to mitochondrial translocator protein 18kDa (TSPO). Despite its convulsant effects, at lower doses Ro5-4864 has proved to be neuroprotective and has become widely used for research into the role of the TSPO protein in neurotoxicity. In vitro studies and rodent models also suggest the possibility of analgesic, antidepressant, cardioprotective, and anti-cancer effects.

Brain positron emission tomography is a form of positron emission tomography (PET) that is used to measure brain metabolism and the distribution of exogenous radiolabeled chemical agents throughout the brain. PET measures emissions from radioactively labeled metabolically active chemicals that have been injected into the bloodstream. The emission data from brain PET are computer-processed to produce multi-dimensional images of the distribution of the chemicals throughout the brain.
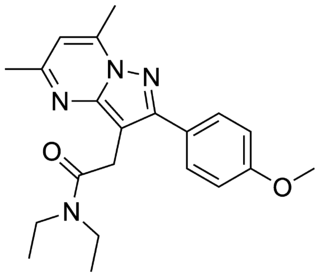
DPA-713 or N,N-diethyl-2-(4-methoxyphenyl)-5,7-dimethylpyrazolo[1,5-a]pyrimidine-3-acetamide is a selective ligand for the translocator protein (TSPO).
The molecular formula C22H27FN4O2 (molar mass: 398.47 g/mol) may refer to:
Crystal C. Watkins Johansson is an American neuroscientist and psychiatrist and associate professor of neuroscience at Johns Hopkins University School of Medicine as well as the director of the Sheppard Pratt Memory Clinic in Neuropsychiatry in Baltimore, Maryland. Johansson was the first Black female Meyerhoff Scholar to obtain an MD/PhD from the University of Maryland, Baltimore County. During her MD/PhD she developed a novel treatment for gastrointestinal in patients with diabetes that led to a patent for a pharmacological compound in 2000. Johansson is a practicing neuropsychiatrist with a focus on geriatric psychiatry and she conducts brain imaging research as well as research on cancer in African American women.
Deuterated etifoxine is a deuterated drug which is under development for the treatment of anxiety disorders and mood disorders. It was originated by GABA Therapeutics and is under development by GABA Therapeutics and ATAI Life Sciences. Deuterated etifoxine is a deuterated form of etifoxine (Stresam) with improved pharmacokinetic properties, for instance a longer elimination half-life and duration of action. Etifoxine has been widely used as an anxiolytic for many decades. Etifoxine and deuterated etifoxine are GABAA receptor positive allosteric modulators (GABAkines) and ligands of the translocator protein (TSPO), both of which may contribute to anxiolytic effects. The TSPO promotes steroidogenesis of inhibitory neurosteroids such as allopregnanolone, which act as potent GABAA receptor positive allosteric modulators, and hence interactions with the TSPO can also indirectly potentiate the GABAA receptor. The precise isotopic substitution of deuterated etifoxine has not yet been disclosed. As of January 2023, deuterated etifoxine is in phase 1 clinical trials for anxiety disorders and preclinical development for mood disorders.
















