
Neurosteroids, also known as neuroactive steroids, are endogenous or exogenous steroids that rapidly alter neuronal excitability through interaction with ligand-gated ion channels and other cell surface receptors. The term neurosteroid was coined by the French physiologist Étienne-Émile Baulieu and refers to steroids synthesized in the brain. The term, neuroactive steroid refers to steroids that can be synthesized in the brain, or are synthesized by an endocrine gland, that then reach the brain through the bloodstream and have effects on brain function. The term neuroactive steroids was first coined in 1992 by Steven Paul and Robert Purdy. In addition to their actions on neuronal membrane receptors, some of these steroids may also exert effects on gene expression via nuclear steroid hormone receptors. Neurosteroids have a wide range of potential clinical applications from sedation to treatment of epilepsy and traumatic brain injury. Ganaxolone, a synthetic analog of the endogenous neurosteroid allopregnanolone, is under investigation for the treatment of epilepsy.

Alpidem, sold under the brand name Ananxyl, is a nonbenzodiazepine anxiolytic medication which was briefly used to treat anxiety disorders but is no longer marketed. It was previously marketed in France, but was discontinued due to liver toxicity. Alpidem is taken by mouth.
The steroidogenic acute regulatory protein, commonly referred to as StAR (STARD1), is a transport protein that regulates cholesterol transfer within the mitochondria, which is the rate-limiting step in the production of steroid hormones. It is primarily present in steroid-producing cells, including theca cells and luteal cells in the ovary, Leydig cells in the testis and cell types in the adrenal cortex.

Cholesterol side-chain cleavage enzyme is commonly referred to as P450scc, where "scc" is an acronym for side-chain cleavage. P450scc is a mitochondrial enzyme that catalyzes conversion of cholesterol to pregnenolone. This is the first reaction in the process of steroidogenesis in all mammalian tissues that specialize in the production of various steroid hormones.

PK-11195 is an isoquinoline carboxamide which binds selectively to the peripheral benzodiazepine receptor (PBR). It is one of the most commonly used PBR ligands due to its high affinity for the PBR in all species, although it is starting to be replaced by newer and more selective ligands.

Etifoxine, sold under the trade name Stresam among others, is a nonbenzodiazepine anxiolytic agent, primarily indicated for short-term management of adjustment disorder, specifically instances of situational depression accompanied by anxiety, such as stress-induced anxiety. Administration is by mouth. Side effects associated with etifoxine use include slight drowsiness, headache, skin eruptions, and allergic reactions. In rare cases, etifoxine has been linked to severe skin and liver toxicity, as well as menstrual bleeding between periods. Unlike benzodiazepines, etifoxine does not cause sedation or lack of coordination. Etifoxine acts as a GABAA receptor positive allosteric modulator and as a ligand for translocator proteins. Both mechanisms are conjectured to contribute to its anxiolytic properties.

Estrogen-related receptor alpha (ERRα), also known as NR3B1, is a nuclear receptor that in humans is encoded by the ESRRA gene. ERRα was originally cloned by DNA sequence homology to the estrogen receptor alpha, but subsequent ligand binding and reporter-gene transfection experiments demonstrated that estrogens did not regulate ERRα. Currently, ERRα is considered an orphan nuclear receptor.

Acyl-CoA-binding protein in humans belongs to the family of Acyl-CoA-binding proteins.

Emapunil is an anxiolytic drug which acts as a selective agonist at the peripheral benzodiazepine receptor, also known as the mitochondrial 18 kDa translocator protein or TSPO. This protein has multiple functions, among which is regulation of steroidogenesis, particularly the production of neuroactive steroids such as allopregnanolone in the brain. In both animal and human trials, emapunil produced fast acting anxiolytic and anti-panic effects, without producing sedation or withdrawal symptoms following cessation of use. Emapunil is also used in its 11C radiolabelled form to map the distribution of TSPO receptors in the brain.
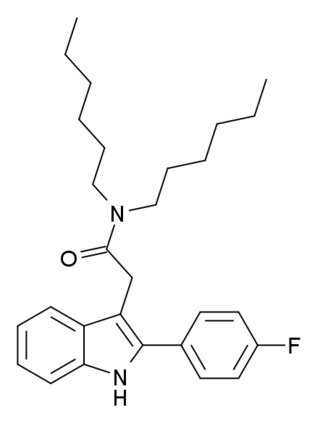
FGIN-1-27 is an anxiolytic drug which acts as a selective agonist at the peripheral benzodiazepine receptor, also known as the mitochondrial 18 kDa translocator protein or TSPO. It is thought to produce anxiolytic effects by stimulating steroidogenesis of neuroactive steroids such as allopregnanolone.

FGIN-1-43 is an anxiolytic drug which acts as a selective agonist at the peripheral benzodiazepine receptor, also known as the mitochondrial 18 kDa translocator protein or TSPO. It is thought to produce anxiolytic effects by stimulating steroidogenesis of neuroactive steroids such as allopregnanolone, and is several times more potent than the related drug FGIN-127.

SSR-180,575 is a drug which acts as a selective agonist at the peripheral benzodiazepine receptor, also known as the mitochondrial 18 kDa translocator protein or TSPO. It has been shown to have neuroprotective and cardioprotective effects and to stimulate steroidogenesis of pregnenolone in the brain, which may be linked to its neuroprotective action.

DAA-1097 is a drug which acts as a potent and selective agonist at the peripheral benzodiazepine receptor, also known as the mitochondrial 18 kDa translocator protein or TSPO, but with no affinity at central benzodiazepine receptors. It has anxiolytic effects in animal studies.
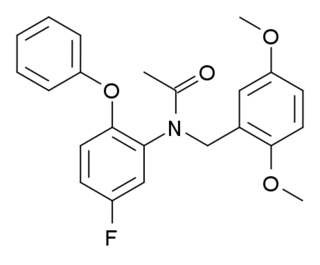
DAA-1106 is a drug which acts as a potent and selective agonist at the peripheral benzodiazepine receptor, also known as the mitochondrial 18 kDa translocator protein or TSPO, but with no affinity at the GABAA receptor. It has anxiolytic effects in animal studies. DAA-1106 has a sub-nanomolar binding affinity (Ki) of 0.28nM, and has been used extensively in its 3H or 11C radiolabelled form to map TSPO in the body and brain, which has proved especially helpful in monitoring the progress of neurodegenerative diseases such as Alzheimer's disease.

Ro5-4864 (4'-chlorodiazepam) is a drug which is a benzodiazepine derivative of diazepam. However unlike most benzodiazepine derivatives, Ro5-4864 lacks affinity for GABAA receptors and lacks typical benzodiazepine effects, instead being sedative yet also convulsant and anxiogenic in effects. Ro5-4864 was found to be a potent ligand for the "peripheral benzodiazepine receptor", later renamed to mitochondrial translocator protein 18kDa (TSPO). Despite its convulsant effects, at lower doses Ro5-4864 has proved to be neuroprotective and has become widely used for research into the role of the TSPO protein in neurotoxicity. In vitro studies and rodent models also suggest the possibility of analgesic, antidepressant, cardioprotective, and anti-cancer effects.
Tryptophan-rich sensory proteins (TspO) are a family of proteins that are involved in transmembrane signalling. In either prokaryotes or mitochondria they are localized to the outer membrane, and have been shown to bind and transport dicarboxylic tetrapyrrole intermediates of the haem biosynthetic pathway. They are associated with the major outer membrane porins and with the voltage-dependent anion channel.

DPA-714 or N,N-diethyl-2-[4-(2-fluoroethoxy)phenyl]-5,7-dimethylpyrazolo[1,5-a]pyrimidine-3-acetamide is a selective ligand for the translocator protein (TSPO) currently under evaluation for several clinical applications. For this reason, a practical, multigram synthetic route for its preparation has been described.
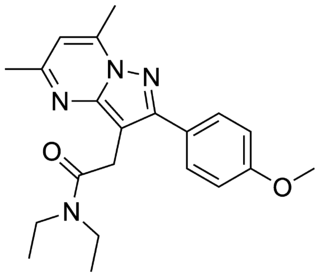
DPA-713 or N,N-diethyl-2-(4-methoxyphenyl)-5,7-dimethylpyrazolo[1,5-a]pyrimidine-3-acetamide is a selective ligand for the translocator protein (TSPO).
A neurosteroidogenesis inhibitor is a drug that inhibits the production of endogenous neurosteroids. Neurosteroids include the excitatory neurosteroids pregnenolone sulfate, dehydroepiandrosterone (DHEA), and dehydroepiandrosterone sulfate (DHEA-S), and the inhibitory neurosteroids allopregnanolone, tetrahydrodeoxycorticosterone (THDOC), and 3α-androstanediol, among others. By inhibiting the synthesis of endogenous neurosteroids, neurosteroidogenesis inhibitors have effects in the central nervous system.

Vassilios Papadopoulos, DPharm, PhD, DSc (hon), born February 18, 1961, in Athens, Greece, is a scholar, researcher, inventor, professor, and university administrator who has served as dean of the USC Alfred E. Mann School of Pharmacy and Pharmaceutical Sciences at the University of Southern California in Los Angeles, California since 2016. Previously, he was the associate vice president and director of the Biomedical Graduate Research Organization at Georgetown University from 2005 to 2007, and the executive director and chief scientific officer of the Research Institute of the McGill University Health Center from 2007 to 2015.




















