Angela Vincent is Emeritus professor at the University of Oxford and a Fellow of Somerville College, Oxford.

In neuroscience, long-term potentiation (LTP) is a persistent strengthening of synapses based on recent patterns of activity. These are patterns of synaptic activity that produce a long-lasting increase in signal transmission between two neurons. The opposite of LTP is long-term depression, which produces a long-lasting decrease in synaptic strength.
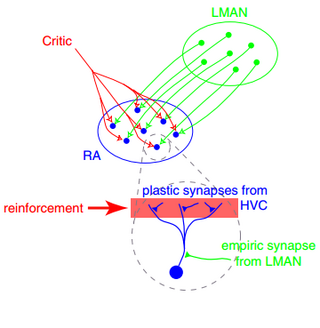
In neuroscience, synaptic plasticity is the ability of synapses to strengthen or weaken over time, in response to increases or decreases in their activity. Since memories are postulated to be represented by vastly interconnected neural circuits in the brain, synaptic plasticity is one of the important neurochemical foundations of learning and memory.
In neuroscience, a silent synapse is an excitatory glutamatergic synapse whose postsynaptic membrane contains NMDA-type glutamate receptors but no AMPA-type glutamate receptors. These synapses are named "silent" because normal AMPA receptor-mediated signaling is not present, rendering the synapse inactive under typical conditions. Silent synapses are typically considered to be immature glutamatergic synapses. As the brain matures, the relative number of silent synapses decreases. However, recent research on hippocampal silent synapses shows that while they may indeed be a developmental landmark in the formation of a synapse, that synapses can be "silenced" by activity, even once they have acquired AMPA receptors. Thus, silence may be a state that synapses can visit many times during their lifetimes.
In neuroscience, homeostatic plasticity refers to the capacity of neurons to regulate their own excitability relative to network activity. The term homeostatic plasticity derives from two opposing concepts: 'homeostatic' and plasticity, thus homeostatic plasticity means "staying the same through change". In the nervous system, neurons must be able to evolve with the development of their constantly changing environment while simultaneously staying the same amidst this change. This stability is important for neurons to maintain their activity and functionality to prevent neurons from carcinogenesis. At the same time, neurons need to have flexibility to adapt to changes and make connections to cope with the ever-changing environment of a developing nervous system.
Epileptogenesis is the gradual process by which a typical brain develops epilepsy. Epilepsy is a chronic condition in which seizures occur. These changes to the brain occasionally cause neurons to fire in an abnormal, hypersynchronous manner, known as a seizure.

Wade G. Regehr is a Professor of Neurobiology at Harvard Medical School's Department of Neurobiology.

The UCL Queen Square Institute of Neurology is an institute within the Faculty of Brain Sciences of University College London (UCL) and is located in London, United Kingdom. Together with the National Hospital for Neurology and Neurosurgery, an adjacent facility with which it cooperates closely, the institute forms a major centre for teaching, training and research in neurology and allied clinical and basic neurosciences.
Christine Elizabeth Holt is a British developmental neuroscientist.
Timothy Vivian Pelham Bliss FRS is a British neuroscientist. He is an adjunct professor at the University of Toronto, and a group leader emeritus at the Francis Crick Institute, London.

Stuart Graham Cull-Candy is a British neuroscientist. He holds the Gaddum Chair of Pharmacology and a personal Chair in Neuroscience at University College London. He is also a member of the Faculty of 1000 and held a Royal Society - Wolfson Research position.

A synaptopathy is a disease of the brain, spinal cord or peripheral nervous system relating to the dysfunction of synapses. This can arise as a result of a mutation in a gene encoding a synaptic protein such as an ion channel, neurotransmitter receptor, or a protein involved in neurotransmitter release. It can also arise as a result of an autoantibody targeting a synaptic protein. Synaptopathies caused by ion channel mutations are also known as synaptic channelopathies. An example is episodic ataxia. Myasthenia gravis is an example of an autoimmune synaptopathy. Some toxins also affect synaptic function. Tetanus toxin and botulinum toxin affect neurotransmitter release. Tetanus toxin can enter the body via a wound, and botulinum toxin can be ingested or administered therapeutically to alleviate dystonia or as cosmetic treatment.
Gene therapy is being studied for some forms of epilepsy. It relies on viral or non-viral vectors to deliver DNA or RNA to target brain areas where seizures arise, in order to prevent the development of epilepsy or to reduce the frequency and/or severity of seizures. Gene therapy has delivered promising results in early stage clinical trials for other neurological disorders such as Parkinson's disease, raising the hope that it will become a treatment for intractable epilepsy.

John O'Keefe, is an American-British neuroscientist, psychologist and a professor at the Sainsbury Wellcome Centre for Neural Circuits and Behaviour and the Research Department of Cell and Developmental Biology at University College London. He discovered place cells in the hippocampus, and that they show a specific kind of temporal coding in the form of theta phase precession. He shared the Nobel Prize in Physiology or Medicine in 2014, together with May-Britt Moser and Edvard Moser; he has received several other awards. He has worked at University College London for his entire career, but also held a part-time chair at the Norwegian University of Science and Technology at the behest of his Norwegian collaborators, the Mosers.

Michael A. Häusser FRS FMedSci is professor of Neuroscience, based in the Wolfson Institute for Biomedical Research at University College London (UCL).

Annette Catherine Dolphin is a Professor of Pharmacology in the Department of Neuroscience, Physiology and Pharmacology at University College London (UCL).
Michael G Hanna is Director of the UCL Institute of Neurology, University College London and professor in clinical neurology and consultant neurologist at the National Hospital for Neurology and Neurosurgery, Queen Square, London, and also Director of the Medical Research Council (MRC) Centre for Neuromuscular Disease.

Maria Fitzgerald is a professor in the Department of Neuroscience at University College London.
Tom Otis is an American researcher, academic and author. He is the Chief Scientific Officer at the Sainsbury Wellcome Centre for Neural Circuits and Behaviour and holds a Professorship in Neuroscience at University College London.
Stephanie Schorge is a Professor of Neuroscience in the Department of Neuroscience, Physiology and Pharmacology at University College London. She is known for her research into mutations that cause neurological diseases.










