
A toxin is a naturally occurring organic poison produced by metabolic activities of living cells or organisms. They occur especially as proteins, often conjugated. The term was first used by organic chemist Ludwig Brieger (1849–1919) and is derived from the word "toxic".
The dinoflagellates are a monophyletic group of single-celled eukaryotes constituting the phylum Dinoflagellata and are usually considered protists. Dinoflagellates are mostly marine plankton, but they also are common in freshwater habitats. Their populations vary with sea surface temperature, salinity, and depth. Many dinoflagellates are photosynthetic, but a large fraction of these are in fact mixotrophic, combining photosynthesis with ingestion of prey.

The alveolates are a group of protists, considered a major clade and superphylum within Eukarya. They are currently grouped with the stramenopiles and Rhizaria among the protists with tubulocristate mitochondria into the SAR supergroup.

Kleptoplasty or kleptoplastidy is a process in symbiotic relationships whereby plastids, notably chloroplasts from algae, are sequestered by the host. The word is derived from Kleptes (κλέπτης) which is Greek for thief. The alga is eaten normally and partially digested, leaving the plastid intact. The plastids are maintained within the host, temporarily continuing photosynthesis and benefiting the host.
Okadaic acid, C44H68O13, is a toxin produced by several species of dinoflagellates, and is known to accumulate in both marine sponges and shellfish. One of the primary causes of diarrhetic shellfish poisoning, okadaic acid is a potent inhibitor of specific protein phosphatases and is known to have a variety of negative effects on cells. A polyketide, polyether derivative of a C38 fatty acid, okadaic acid and other members of its family have shined light upon many biological processes both with respect to dinoflagellete polyketide synthesis as well as the role of protein phosphatases in cell growth.

Noctiluca scintillans is a marine species of dinoflagellate that can exist in a green or red form, depending on the pigmentation in its vacuoles. It can be found worldwide, but its geographical distribution varies depending on whether it is green or red. This unicellular microorganism is known for its ability to bioluminesce, giving the water a bright blue glow seen at night. However, blooms of this species can be responsible for environmental hazards, such as toxic red tides. They may also be an indicator of anthropogenic eutrophication.

Paralytic shellfish poisoning (PSP) is one of the four recognized syndromes of shellfish poisoning, which share some common features and are primarily associated with bivalve mollusks. These shellfish are filter feeders and accumulate neurotoxins, chiefly saxitoxin, produced by microscopic algae, such as dinoflagellates, diatoms, and cyanobacteria. Dinoflagellates of the genus Alexandrium are the most numerous and widespread saxitoxin producers and are responsible for PSP blooms in subarctic, temperate, and tropical locations. The majority of toxic blooms have been caused by the morphospecies Alexandrium catenella, Alexandrium tamarense, Gonyaulax catenella and Alexandrium fundyense, which together comprise the A. tamarense species complex. In Asia, PSP is mostly associated with the occurrence of the species Pyrodinium bahamense.
Diarrheic shellfish poisoning (DSP) is one of the four recognized symptom types of shellfish poisoning, alongside paralytic shellfish poisoning, neurotoxic shellfish poisoning and amnesic shellfish poisoning. As the name suggests, it mainly manifests as diarrhea. Abdominal pain, nausea and vomiting may also occur.

Karenia is a genus that consists of unicellular, photosynthetic, planktonic organisms found in marine environments. The genus currently consists of 12 described species. They are best known for their dense toxic algal blooms and red tides that cause considerable ecological and economical damage; some Karenia species cause severe animal mortality. One species, Karenia brevis, is known to cause respiratory distress and neurotoxic shellfish poisoning (NSP) in humans.

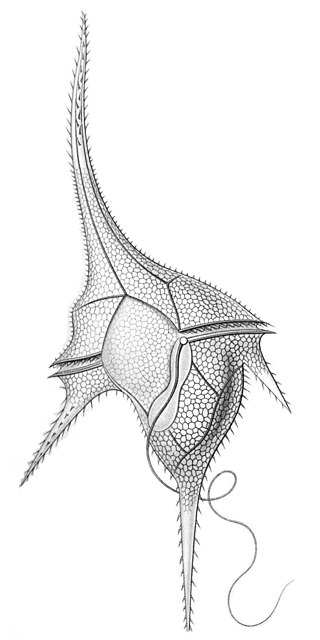
Ornithocercus is a genus of planktonic dinoflagellate that is known for its complex morphology that features considerable lists growing from its thecal plates, giving an attractive appearance. Discovered in 1883, this genus has a small number of species currently categorized but is widespread in tropical and sub-tropical oceans. The genus is marked by exosymbiotic bacteria gardens under its lists, the inter-organismal dynamics of which are a current field of research. As they reside only in warm water, the genus has been used as a proxy for climate change and has potential to be an indicator species for environmental change if found in novel environments.
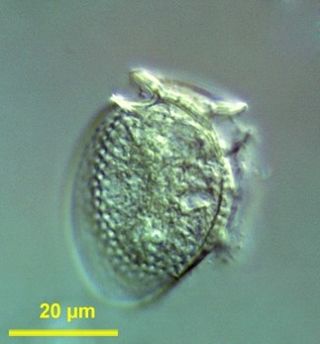
Dinophysis is a genus of dinoflagellates common in tropical, temperate, coastal and oceanic waters. It was first described in 1839 by Christian Gottfried Ehrenberg.
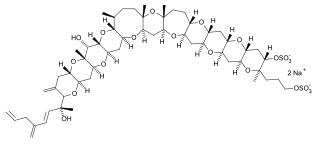
Yessotoxins are a group of lipophilic, sulfur bearing polyether toxins that are related to ciguatoxins. They are produced by a variety of dinoflagellates, most notably Lingulodinium polyedrum and Gonyaulax spinifera.

Dinophysis norvegica is a species of dinoflagellate most commonly associated with diarrheal shellfish poisoning.
Mesodinium chamaeleon is a ciliate of the genus Mesodinium. It is known for being able to consume and maintain algae endosymbiotically for days before digesting the algae. It has the ability to eat red and green algae, and afterwards using the chlorophyll granules from the algae to generate energy, turning itself from being a heterotroph into an autotroph. The species was discovered in January 2012 outside the coast of Nivå, Denmark by professor Øjvind Moestrup.
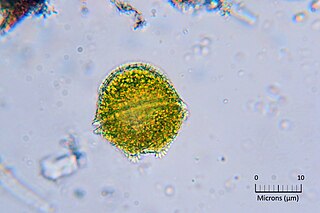
Alexandrium is a genus of dinoflagellates. It contains some of the dinoflagellate species most harmful to humans, because it produces toxic harmful algal blooms (HAB) that cause paralytic shellfish poisoning (PSP) in humans. There are about 30 species of Alexandrium that form a clade, defined primarily on morphological characters in their thecal plates.
Dinotoxins are a group of toxins which are produced by flagellate, aquatic, unicellular protists called dinoflagellates. Dinotoxin was coined by Hardy and Wallace in 2012 as a general term for the variety of toxins produced by dinoflagellates. Dinoflagellates are an enormous group of marine life, with much diversity. With great diversity comes many different toxins, however, there are a few toxins that multiple species have in common.

Mesodinium rubrum is a species of ciliates. It constitutes a plankton community and is found throughout the year, most abundantly in spring and fall, in coastal areas. Although discovered in 1908, its scientific importance came into light in the late 1960s when it attracted scientists by the recurrent red colouration it caused by forming massive blooms, that cause red tides in the oceans.

Dinophysis acuta is a species of flagellated planktons belonging to the genus Dinophysis. It is one of the few unusual photosynthetic protists that acquire plastids from algae by endosymbiosis. By forming massive blooms, particularly in late summer and spring, it causes red tides. It produces toxic substances and the red tides cause widespread infection of seafood, particularly crabs and mussels. When infected animals are consumed, severe diarrhoea occurs. The clinical symptom is called diarrhetic shellfish poisoning. The main chemical toxins were identified in 2006 as okadaic acid and pectenotoxins. They can produce non-fatal or fatal amounts of toxins in their predators, which can become toxic to humans.

Dinoflagellates are eukaryotic plankton, existing in marine and freshwater environments. Previously, dinoflagellates had been grouped into two categories, phagotrophs and phototrophs. Mixotrophs, however include a combination of phagotrophy and phototrophy. Mixotrophic dinoflagellates are a sub-type of planktonic dinoflagellates and are part of the phylum Dinoflagellata. They are flagellated eukaryotes that combine photoautotrophy when light is available, and heterotrophy via phagocytosis. Dinoflagellates are one of the most diverse and numerous species of phytoplankton, second to diatoms.

Mesodinium is a genus of ciliates that are widely distributed and are abundant in marine and brackish waters.
















