
Polychaeta is a paraphyletic class of generally marine annelid worms, commonly called bristle worms or polychaetes. Each body segment has a pair of fleshy protrusions called parapodia that bear many bristles, called chaetae, which are made of chitin. More than 10,000 species are described in this class. Common representatives include the lugworm and the sandworm or clam worm Alitta.

A chaeta or cheta is a chitinous bristle or seta found on annelid worms, although the term is also frequently used to describe similar structures in other invertebrates such as arthropods. Polychaete annelids are named for their chaetae. In Polychaeta, chaetae are found as bundles on the parapodia, paired appendages on the side of the body. The chaetae are epidermal, extracellular structures, and clearly visible in most polychaetes. They are probably the best-studied structures in these animals. Segments bearing chaetae are called chaetigers.

The Clitellata are a class of annelid worms, characterized by having a clitellum – the 'collar' that forms a reproductive cocoon during part of their life cycles. The clitellates comprise around 8,000 species. Unlike the class of Polychaeta, they do not have parapodia and their heads are less developed.

Australonuphis, commonly called Australian beach worms, are a genus of polychaetous annelid of the family Onuphidae that inhabit the intertidal zone of coastal beaches and are attracted to the surface by the stimulus of food. They are sought by anglers to be used as bait for fishing. Some species can grow more than two metres in length. They are blind but have a very good sense of smell, and eat decaying meat, fish and seaweeds that have washed to shore.

Serpula is a genus of sessile, marine annelid tube worms that belongs to the family Serpulidae. Serpulid worms are very similar to tube worms of the closely related sabellid family, except that the former possess a cartilaginous operculum that occludes the entrance to their protective tube after the animal has withdrawn into it. The most distinctive feature of worms of the genus Serpula is their colorful fan-shaped "crown". The crown, used by these animals for respiration and alimentation, is the structure that is most commonly seen by scuba divers and other casual observers.

Alitta succinea is a species of marine annelid in the family Nereididae. It has been recorded throughout the North West Atlantic, as well as in the Gulf of Maine and South Africa.

Capitella teleta is a small, cosmopolitan, segmented annelid worm. It is a well-studied invertebrate, which has been cultured for use in laboratories for over 30 years. C. teleta is the first marine polychaete to have its genome sequenced.

Pomatoceros triqueter is a species of tube-building annelid worm in the class Polychaeta. It is common on the north eastern coasts of the Atlantic Ocean and in the Mediterranean Sea.

Sabellastarte spectabilis is a species of benthic marine polychaete worm in the Sabellidae family. It is commonly known as the feather duster worm, feather duster or fan worm. It is native to tropical waters of the Indo-Pacific but has spread to other parts of the world. It is popular in aquariums because of its distinctive appearance and its ability to remove organic particles and improve water quality.
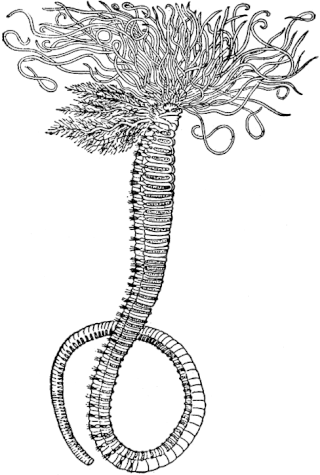
Amphitrite ornata or ornate worm, is a species of marine polychaete worm in the family Terebellidae.

Cirratulus cirratus is a species of marine polychaete worm in the family Cirratulidae. It occurs in the littoral and sub-littoral zones of the Atlantic Ocean.
The Onuphidae are a family of polychaete worms.

Diopatra is a genus of polychaete worms in the family Onuphidae.

The annelids, also known as the segmented worms, are a large phylum, with over 22,000 extant species including ragworms, earthworms, and leeches. The species exist in and have adapted to various ecologies – some in marine environments as distinct as tidal zones and hydrothermal vents, others in fresh water, and yet others in moist terrestrial environments.
Prosphaerosyllis battiri is a species belonging to the phylum Annelida, a group known as the segmented worms. The species name comes from an Aboriginal word, battiri, meaning "rough". Prosphaerosyllis battiri is a species characterized by having only partially fused palps, an unretracted prostomium on its peristomium or showing only slight retraction, the shape of its dorsal cirri and its arrangement of papillae, being numerous anteriorly while less numerous posteriorly. It resembles Prosphaerosyllis semiverrucosa, but its arrangement of dorsal papillae is reversed.
Ophryotrocha craigsmithi is a species of polychaete worm. O. craigsmithi is named after Craig R. Smith. This species is similar to Palpiphitime lipovskyae and O. Platykephale, among others, in having branchial structures dorsally and ventrally. It differs from O. platykephale in the shape of its prostomium and parapodia. Palpiphitime lipovskyae has jaws of both P- and K-type, while no specimens of O. craigsmithi have been found with K-type jaws thus far. Ophryotrocha craigsmithi differs from P. lipovskyae genetically, but also by the presence of a prominent ventral chaetal lobe with a bulging simple chaeta in the former.

Diopatra cuprea, commonly known as the plumed worm, decorator worm or sometimes ornate worm, is a species of polychaete worm in the family Onuphidae, first described by the French entomologist Louis Augustin Guillaume Bosc in 1802. It is native to the northwestern Atlantic Ocean, the Caribbean Sea and the Gulf of Mexico.

Eulagisca gigantea is a species of scale worm. This species is specifically found in the deep-sea in cold waters like the Antarctic Ocean. The scale worms are named for the elytra on their surface that look like scales.

Poeobius is a genus of marine polychaete worm. It contains the single species Poeobius meseres, or balloon worm. This is a common and abundant resident in the midwater around the mesopelagic and bathypelagic zones, especially in Monterey Bay. They can be found at around 300-2,500 m depth from Japan to Alaska to the Gulf of California, and have also been reported in South America.

Buskiella is a genus of pelagic polychaete annelids placed either in the family Flotidae or Flabelligeridae. In appearance, they are generally bluish or yellowish, depending on lighting conditions, and live exclusively in very deep water. They move by swinging their bodies from side to side, "rowing with [their] bristles." Species have nine to eleven chaetigers.
















