
Chemiluminescence is the emission of light (luminescence) as the result of a chemical reaction. There may also be limited emission of heat. Given reactants A and B, with an excited intermediate ◊,
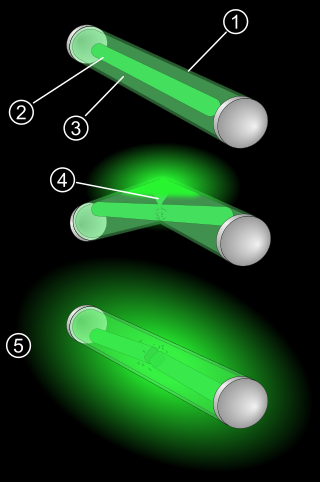
A glow stick, also known as a light stick, chem light, light wand, light rod, and rave light, is a self-contained, short-term light-source. It consists of a translucent plastic tube containing isolated substances that, when combined, make light through chemiluminescence. The light cannot be turned off and can be used only once. The used tube is then thrown away. Glow sticks are often used for recreation, such as for events, camping, outdoor exploration, and concerts. Glow sticks are also used for light in military and emergency services applications. Industrial uses include marine, transportation, and mining.

Phosphorescence is a type of photoluminescence related to fluorescence. When exposed to light (radiation) of a shorter wavelength, a phosphorescent substance will glow, absorbing the light and reemitting it at a longer wavelength. Unlike fluorescence, a phosphorescent material does not immediately reemit the radiation it absorbs. Instead, a phosphorescent material absorbs some of the radiation energy and reemits it for a much longer time after the radiation source is removed.

Luminol (C8H7N3O2) is a chemical that exhibits chemiluminescence, with a blue glow, when mixed with an appropriate oxidizing agent. Luminol is a white-to-pale-yellow crystalline solid that is soluble in most polar organic solvents, but insoluble in water.

In organic chemistry, sulfonic acid refers to a member of the class of organosulfur compounds with the general formula R−S(=O)2−OH, where R is an organic alkyl or aryl group and the S(=O)2(OH) group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound is the parent sulfonic acid, HS(=O)2(OH), a tautomer of sulfurous acid, S(=O)(OH)2. Salts or esters of sulfonic acids are called sulfonates.

In organic chemistry, organic peroxides are organic compounds containing the peroxide functional group. If the R′ is hydrogen, the compounds are called hydroperoxides, which are discussed in that article. The O−O bond of peroxides easily breaks, producing free radicals of the form RO•. Thus, organic peroxides are useful as initiators for some types of polymerization, such as the acrylic, unsaturated polyester, and vinyl ester resins used in glass-reinforced plastics. MEKP and benzoyl peroxide are commonly used for this purpose. However, the same property also means that organic peroxides can explosively combust. Organic peroxides, like their inorganic counterparts, are often powerful bleaching agents.

Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group [R−N+≡N]X− where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide.
In organic chemistry, an azo coupling is an organic reaction between a diazonium compound and another aromatic compound that produces an azo compound. In this electrophilic aromatic substitution reaction, the aryldiazonium cation is the electrophile and the activated carbon act as a nucleophile. In most cases, including the examples below, the diazonium compound is also aromatic.

In organic chemistry, a carbonate ester is an ester of carbonic acid. This functional group consists of a carbonyl group flanked by two alkoxy groups. The general structure of these carbonates is R−O−C(=O)−O−R' and they are related to esters, ethers and also to the inorganic carbonates.

Polyester is a category of polymers that contain the ester functional group in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing.

Bis[2,4,5-trichloro-6-(pentyloxycarbonyl)phenyl]oxalate is a solid ester whose oxidation products are responsible for the chemiluminescence in a glowstick. It can be synthesized by reacting 2-carbopentoxy-3,5,6-trichlorophenol with oxalyl chloride.

Dimethyl carbonate (DMC) is an organic compound with the formula OC(OCH3)2. It is a colourless, flammable liquid. It is classified as a carbonate ester. This compound has found use as a methylating agent and more recently as a solvent that is exempt from the restrictions placed on most volatile organic compounds (VOCs) in the United States. Dimethyl carbonate is often considered to be a green reagent.
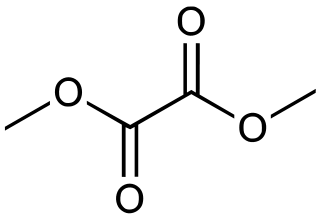
Dimethyl oxalate is an organic compound with the formula (CO2CH3)2. It is the dimethyl ester of oxalic acid. Dimethyl oxalate is a colorless or white solid that is soluble in water.

Electrochemiluminescence or electrogenerated chemiluminescence (ECL) is a kind of luminescence produced during electrochemical reactions in solutions. In electrogenerated chemiluminescence, electrochemically generated intermediates undergo a highly exergonic reaction to produce an electronically excited state that then emits light upon relaxation to a lower-level state. This wavelength of the emitted photon of light corresponds to the energy gap between these two states. ECL excitation can be caused by energetic electron transfer (redox) reactions of electrogenerated species. Such luminescence excitation is a form of chemiluminescence where one/all reactants are produced electrochemically on the electrodes.

Diphenyl ether is the organic compound with the formula (C6H5)2O. It is a colorless, low-melting solid. This, the simplest diaryl ether, has a variety of niche applications.

Peroxyoxalates are esters initially formed by the reaction of hydrogen peroxide with oxalate diesters or oxalyl chloride, with or without base, although the reaction is much faster with base:

Diphenyl carbonate is the organic compound with the formula (C6H5O)2CO. It is classified as an acyclic carbonate ester. It is a colorless solid. It is both a monomer in combination with bisphenol A in the production of polycarbonate polymers and a product of the decomposition of polycarbonates.

The chemical compound 1,2-dioxetanedione, or 1,2-dioxacyclobutane-3,4-dione, often called peroxyacid ester, is an unstable oxide of carbon (an oxocarbon) with formula C2O4. It can be viewed as a double ketone of 1,2-dioxetane (1,2-dioxacyclobutane), or a cyclic dimer of carbon dioxide.

TCPO, or bis(2,4,6-trichlorophenyl) oxalate, is a chemical used in some types of glow sticks.

o-Cresolphthalein is a phthalein dye used as a pH indicator in titrations. It is insoluble in water but soluble in ethanol. Its solution is colourless below pH 8.2, and purple above 9.8. Its molecular formula is C22H18O4. It is used medically to determine calcium levels in the human body, or to synthesize polyamides or polyimides.





















