
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula CnH2n−2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic.
The Sonogashira reaction is a cross-coupling reaction used in organic synthesis to form carbon–carbon bonds. It employs a palladium catalyst as well as copper co-catalyst to form a carbon–carbon bond between a terminal alkyne and an aryl or vinyl halide.
In organic chemistry, arynes and benzynes are a class of highly reactive chemical species derived from an aromatic ring by removal of two substituents. Arynes are examples of didehydroarenes, although 1,3- and 1,4-didehydroarenes are also known. Arynes are examples of alkynes under high strain.
An alkyne trimerisation is a [2+2+2] cycloaddition reaction in which three alkyne units react to form a benzene ring. The reaction requires a metal catalyst. The process is of historic interest as well as being applicable to organic synthesis. Being a cycloaddition reaction, it has high atom economy. Many variations have been developed, including cyclisation of mixtures of alkynes and alkenes as well as alkynes and nitriles.
The azide-alkyne Huisgen cycloaddition is a 1,3-dipolar cycloaddition between an azide and a terminal or internal alkyne to give a 1,2,3-triazole. Rolf Huisgen was the first to understand the scope of this organic reaction. American chemist Karl Barry Sharpless has referred to this cycloaddition as "the cream of the crop" of click chemistry and "the premier example of a click reaction".

Copper(II) acetate, also referred to as cupric acetate, is the chemical compound with the formula Cu(OAc)2 where AcO− is acetate (CH
3CO−
2). The hydrated derivative, Cu2(OAc)4(H2O)2, which contains one molecule of water for each copper atom, is available commercially. Anhydrous copper(II) acetate is a dark green crystalline solid, whereas Cu2(OAc)4(H2O)2 is more bluish-green. Since ancient times, copper acetates of some form have been used as fungicides and green pigments. Today, copper acetates are used as reagents for the synthesis of various inorganic and organic compounds. Copper acetate, like all copper compounds, emits a blue-green glow in a flame.

Benzothiophene is an aromatic organic compound with a molecular formula C8H6S and an odor similar to naphthalene (mothballs). It occurs naturally as a constituent of petroleum-related deposits such as lignite tar. Benzothiophene has no household use. In addition to benzo[b]thiophene, a second isomer is known: benzo[c]thiophene.
1,2,3-Triazole is one of a pair of isomeric chemical compounds with molecular formula C2H3N3, called triazoles, which have a five-membered ring of two carbon atoms and three nitrogen atoms. 1,2,3-Triazole is a basic aromatic heterocycle.

Titanocene dichloride is the organotitanium compound with the formula (η5-C5H5)2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air. It shows antitumour activity and was the first non-platinum complex to undergo clinical trials as a chemotherapy drug.

Schwartz's reagent is the common name for the organozirconium compound with the formula (C5H5)2ZrHCl, sometimes called zirconocene hydrochloride or zirconocene chloride hydride, and is named after Jeffrey Schwartz, a chemistry professor at Princeton University. This metallocene is used in organic synthesis for various transformations of alkenes and alkynes.
The Cadiot–Chodkiewicz coupling in organic chemistry is a coupling reaction between a terminal alkyne and a haloalkyne catalyzed by a copper(I) salt such as copper(I) bromide and an amine base. The reaction product is a 1,3-diyne or di-alkyne.

Organocopper chemistry is the study of the physical properties, reactions, and synthesis of organocopper compounds, which are organometallic compounds containing a carbon to copper chemical bond. They are reagents in organic chemistry.
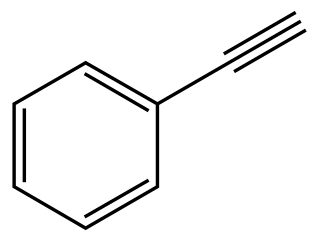
Phenylacetylene is an alkyne hydrocarbon containing a phenyl group. It exists as a colorless, viscous liquid. In research, it is sometimes used as an analog for acetylene; being a liquid, it is easier to handle than acetylene gas.

A boronic acid is an organic compound related to boric acid in which one of the three hydroxyl groups is replaced by an alkyl or aryl group. As a compound containing a carbon–boron bond, members of this class thus belong to the larger class of organoboranes.
The Kulinkovich reaction describes the organic synthesis of substituted cyclopropanols through reaction of esters with dialkyldialkoxytitanium reagents, which are generated in situ from Grignard reagents containing a hydrogen in beta-position and titanium(IV) alkoxides such as titanium isopropoxide. This reaction was first reported by Oleg Kulinkovich and coworkers in 1989.

Organonickel chemistry is a branch of organometallic chemistry that deals with organic compounds featuring nickel-carbon bonds. They are used as a catalyst, as a building block in organic chemistry and in chemical vapor deposition. Organonickel compounds are also short-lived intermediates in organic reactions. The first organonickel compound was nickel tetracarbonyl Ni(CO)4, reported in 1890 and quickly applied in the Mond process for nickel purification. Organonickel complexes are prominent in numerous industrial processes including carbonylations, hydrocyanation, and the Shell higher olefin process.
The Glaser coupling is a type of coupling reaction. It is by far one of the oldest coupling reactions and is based on copper compounds like copper(I) chloride or copper(I) bromide and an additional oxidant like air. The base used in the original research paper is ammonia and the solvent is water or an alcohol. The reaction was first reported by Carl Andreas Glaser in 1869. He suggested the following process on his way to diphenylbutadiyne:
Organogold chemistry is the study of compounds containing gold–carbon bonds. They are studied in academic research, but have not received widespread use otherwise. The dominant oxidation states for organogold compounds are I with coordination number 2 and a linear molecular geometry and III with CN = 4 and a square planar molecular geometry.

The thiol-yne reaction is an organic reaction between a thiol and an alkyne. The reaction product is an alkenyl sulfide. The reaction was first reported in 1949 with thioacetic acid as reagent and rediscovered in 2009. It is used in click chemistry and in polymerization, especially with dendrimers.

Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. Copper compounds, whether organic complexes or organometallics, promote or catalyse numerous chemical and biological processes.











