This article needs editing to comply with Wikipedia's Manual of Style.(August 2024) |
Dipshikha Chakravortty | |
|---|---|
| Born | Jabalpur, Madhya Pradesh, India |
| Alma mater | |
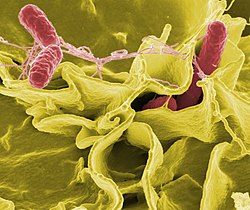
| Known for | Studies on Salmonella, antibacterial resistance |
| Awards |
|
| Scientific career | |
| Fields | |
| Institutions | |
| Doctoral advisor |
|
Dipshikha Chakravortty is an Indian microbiologist, molecular pathologist and a professor at the department of Microbiology and Cell Biology at the Indian Institute of Science. Known for her studies on Salmonella and antibacterial resistance, Chakravortty is an elected fellow of The World Academy of Sciences (FTWAS), the National Academy of Sciences, India, the Indian Academy of Sciences and the Indian National Science Academy. The Department of Biotechnology of the Government of India awarded her the National Bioscience Award for Career Development, one of the highest Indian science awards, for her contributions to biosciences, in 2010. [1]