Design
All triptans have an indole structure identical to the neurotransmitter 5-HT. Classic triptan structure contain side chain on the indole ring, and a basic nitrogen in a similar distance from the indole structure. The main structural difference of the triptans is the position of the sulfonamide and the side chain attached to it (see figure 1 and table 1). Rizatriptan and zolmitriptan have instead of a sulfonamide a triazole and 2-oxazolidone respectively. Another exception to the classic structure is seen on eletriptan where the nitrogen-alkyl chain connected to the indole ring is replaced with a dimethyl-pyrrolidine, and in naratriptan where the nitrogen-alkyl chain is replaced with a 1-methyl-piperidine ring.
One of the frovatriptan side chains forms an additional ring with the indole, resulting in a carbazole ring system.
Structures of the triptans
| Triptan | R1 | R2 | Triptan | R1 | R2 |
|---|---|---|---|---|---|
| Sumatriptan | 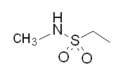 |  | Eletriptan | 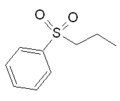 |  |
| Rizatriptan |  |  | Naratriptan |  |  |
| Almotriptan |  |  | Frovatriptan |  |  |
| Zolmitriptan |  |  | – | – | – |
The 5-HT1B/D pharmacophore


5-HT1B and 5-HT1D receptors are considered very similar, they share amino acid homology and their ligands expose similar binding properties thus they have similar pharmacophore. The pharmacophore model for these receptors ligands is qualitative and defines the relative positions of important groups. It is defined with following five main features: an aromatic group (usually the indole), protonated amine (a donor of hydrogen bond), acceptor of hydrogen bond, additional hydrogen bond site (both donor and acceptor) and hydrophobic region located between both hydrogen bond sites, see figure 2. [11] [14] The main binding points were concluded to be the protonated amine and the hydrogen bond site. It was observed that the double bond region in the indole was necessary for the agonism in this series of compounds. Figure 3 shows how different drugs fit the pharmacophore, with a C and N linked analogues of 5-HT1D agonist. The marked sites on the figure are responsible for the affinity. [14] [15] The pharmacophore can be characterized as amphipathic, that means that the structure has both hydrophobic and hydrophilic groups. [16]
Relevant structural features of triptans and binding to the receptor
Triptan structures were designed from the structure of 5-HT to attain affinity to 5-HT receptors, hence the identical indole structure. The hydroxyl group (-OH) on the hexane of the indole core and the alkyl-amine side chain on position C3 on 5-HT have been replaced with other compounds, such as sulfonamides or azol-ring structured derivatives and different amine-alkyl side chains. An electro-negative group can form a hydrogen bond with Thr in the pocket of the receptor. Sulfonamide derivatives attached to the hexane ring of the indole structure have electro-negative properties, as well as the triazole and 2-oxazolidone on rizatriptan and zolmitriptan respectively. This can increase binding ability of the compound and the efficacy, especially with the 5-HT1D receptor. [11]

A schematic drawing of the binding of sumatriptan to 5-HT1D receptor can be seen in figure 4. One study [11] showed that sumatriptan fits better in the binding site of the receptor when the side chain with the protonated nitrogen atom is folded back over the indole structure. This alignment contributes to the hydrogen bonding between the nitrogen in the sulfonamide and the Ser138 in the binding site. It is also favorable to the formation of the hydrogen bond between the oxygen of the sulfonamide and Thr202. Other binding in the pocket of the binding site occurs with the nitrogen atom in the pentene ring of the indole structure of the triptan and the amino acid Ser352. This energetically favorable position of the agonist makes it possible for additional binding of the ligand to other Ser in the binding site, along with additional anchoring between Phe in the pocket of the binding site and the indole of the agonist. The binding of Phe and the triptan is caused by π stacking interactions of the indole and amino acid and an additional effect on this interaction is because of dispersive effect of amino acid leucine (Leu; not shown in figure 4). The amino acids Trp343 and Tyr346 both have electron rich π-systems in their aromatic structures. With their position in the binding site they create a sort of aromatic cage around the protonated nitrogen atom of the side chain on position C3 on the triptans (this nitrogen atom is protonated at physiological condition), and thereby stabilizes the ion bond the nitrogen atom has formed with a carboxylate on aspartic acid. Side chains of the surrounding amino acids can have an effect on the binding of the nitrogen atom, mainly three Phe can affect the methyl groups bound to the nitrogen atom (not shown in figure 4). [11] [12] [13]
Eletriptan has higher affinity for the receptor, which is probably a result of the bulky substituents of the structure.[ citation needed ] The amine is protonated at physiological pH condition, triggering better uptake. [16] [17] The uptake rate of the agonist is different depending on whether the amine in R2 is primary, secondary or tertiary but the latter seem to give the best results. For the R1 substituent an electron rich sulfonamide groups and amide group has shown the best results in receptor binding and activity. [16] It has been observed that a relationship is between absorption and molecular size hence larger hydrophilic molecules tended to have poor absorption. A small R1 substituent is necessary to maintain the rapid oral bioavailability of triptans. [15]
By placing an electron-withdrawing group or large group on position C2 on the indole structure the 5-HT agonist is conversed into an antagonist. This is thought to be because the indole ring is unable to occupy the aromatic part of the binding site. [12]

