In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomerization. When the isomerization occurs intramolecularly it may be called a rearrangement reaction.

Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet, visible (400–750 nm), or infrared radiation (750–2500 nm).

Ronald George Wreyford Norrish FRS was a British chemist who was awarded the Nobel Prize in Chemistry in 1967.
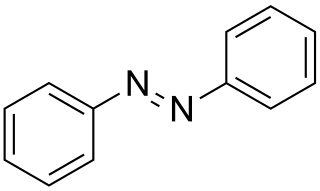
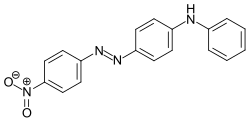
Azobenzene is a photoswitchable chemical compound composed of two phenyl rings linked by a N=N double bond. It is the simplest example of an aryl azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of similar compounds. These azo compounds are considered as derivatives of diazene (diimide), and are sometimes referred to as 'diazenes'. The diazenes absorb light strongly and are common dyes. Different classes of azo dyes exist, most notably the ones substituted with heteroaryl rings.
In chemistry, photoisomerization is a form of isomerization induced by photoexcitation. Both reversible and irreversible photoisomerizations are known for photoswitchable compounds. The term "photoisomerization" usually, however, refers to a reversible process.

In chemistry, photocatalysis is the acceleration of a photoreaction in the presence of a photocatalyst, the excited state of which "repeatedly interacts with the reaction partners forming reaction intermediates and regenerates itself after each cycle of such interactions." In many cases, the catalyst is a solid that upon irradiation with UV- or visible light generates electron–hole pairs that generate free radicals. Photocatalysts belong to three main groups; heterogeneous, homogeneous, and plasmonic antenna-reactor catalysts. The use of each catalysts depends on the preferred application and required catalysis reaction.

Dichlorine monoxide is an inorganic compound with the molecular formula Cl2O. It was first synthesised in 1834 by Antoine Jérôme Balard, who along with Gay-Lussac also determined its composition. In older literature it is often referred to as chlorine monoxide, which can be a source of confusion as that name now refers to the ClO• radical.

Maleic acid or cis-butenedioic acid is an organic compound that is a dicarboxylic acid, a molecule with two carboxyl groups. Its chemical formula is HO2CCH=CHCO2H. Maleic acid is the cis isomer of butenedioic acid, whereas fumaric acid is the trans isomer. Maleic acid is mainly used as a precursor to fumaric acid, and relative to its parent maleic anhydride, which has many applications.

Photochromism is the reversible change of color upon exposure to light. It is a transformation of a chemical species (photoswitch) between two forms by the absorption of electromagnetic radiation (photoisomerization), where the two forms have different absorption spectra.
Radiation chemistry is a subdivision of nuclear chemistry which studies the chemical effects of ionizing radiation on matter. This is quite different from radiochemistry, as no radioactivity needs to be present in the material which is being chemically changed by the radiation. An example is the conversion of water into hydrogen gas and hydrogen peroxide.
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by absorption of light or photons. It is defined as the interaction of one or more photons with one target molecule that dissociates into two fragments.

Molecular tagging velocimetry (MTV) is a specific form of flow velocimetry, a technique for determining the velocity of currents in fluids such as air and water. In its simplest form, a single "write" laser beam is shot once through the sample space. Along its path an optically induced chemical process is initiated, resulting in the creation of a new chemical species or in changing the internal energy state of an existing one, so that the molecules struck by the laser beam can be distinguished from the rest of the fluid. Such molecules are said to be "tagged".
The Barton reaction, also known as the Barton nitrite ester reaction, is a photochemical reaction that involves the photolysis of an alkyl nitrite to form a δ-nitroso alcohol.

In polymer chemistry photo-oxidation is the degradation of a polymer surface due to the combined action of light and oxygen. It is the most significant factor in the weathering of plastics. Photo-oxidation causes the polymer chains to break, resulting in the material becoming increasingly brittle. This leads to mechanical failure and, at an advanced stage, the formation of microplastics. In textiles the process is called phototendering.

Nitrogen trioxide or nitrate radical is an oxide of nitrogen with formula NO
3, consisting of three oxygen atoms covalently bound to a nitrogen atom. This highly unstable blue compound has not been isolated in pure form, but can be generated and observed as a short-lived component of gas, liquid, or solid systems.

Brevianamides are indole alkaloids that belong to a class of naturally occurring 2,5-diketopiperazines produced as secondary metabolites of fungi in the genus Penicillium and Aspergillus. Structurally similar to paraherquamides, they are a small class compounds that contain a bicyclo[2.2.2]diazoctane ring system. One of the major secondary metabolites in Penicillium spores, they are responsible for inflammatory response in lung cells.
Photoelectrochemical processes are processes in photoelectrochemistry; they usually involve transforming light into other forms of energy. These processes apply to photochemistry, optically pumped lasers, sensitized solar cells, luminescence, and photochromism.
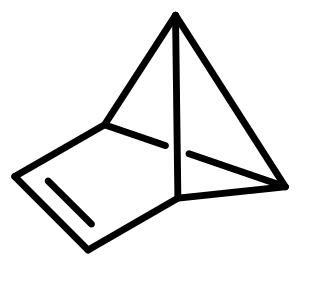
Benzvalene is an organic compound and one of several isomers of benzene. It was first synthesized in 1967 by K. E. Wilzbach et al. via photolysis of benzene and the synthesis was later improved by Thomas J. Katz et al.

Edward Meredith Burgess was an American chemist. He specialized in organic chemistry with an emphasis on methodology, structure, and photochemistry. He is best known for the Burgess reagent that is used for selective dehydration of alcohols.

Tetramethylethylene is a hydrocarbon with the formula Me2C=CMe2 (Me = methyl). A colorless liquid, it is the simplest tetrasubstituted alkene.