
In organic chemistry, a ketone is an organic compound with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.
In chemistry, a pentose is a monosaccharide with five carbon atoms. The chemical formula of many pentoses is C
5H
10O
5, and their molecular weight is 150.13 g/mol.

In organic chemistry, an aldehyde is an organic compound containing a functional group with the structure R−CH=O. The functional group itself can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology.

Redox is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. The oxidation and reduction processes occur simultaneously in the chemical reaction.
Benedict's reagent is a chemical reagent and complex mixture of sodium carbonate, sodium citrate, and copper(II) sulfate pentahydrate. It is often used in place of Fehling's solution to detect the presence of reducing sugars and other reducing substances. Tests that use this reagent are called Benedict's tests. A positive result of Benedict's test is indicated by a color change from clear blue to brick-red with a precipitate.
Chromic acid is jargon for a solution formed by the addition of sulfuric acid to aqueous solutions of dichromate. It consists at least in part of chromium trioxide.

Copper(I) oxide or cuprous oxide is the inorganic compound with the formula Cu2O. It is one of the principal oxides of copper, the other being copper(II) oxide or cupric oxide (CuO).The compound can appear either yellow or red, depending on the size of the particles. Cuprous oxide is found as the mineral cuprite. It is a component of some antifouling paints, but also has other applications including some that exploit its property as a semiconductor.
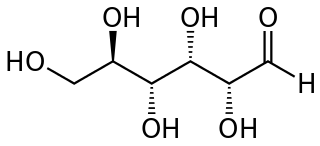
A reducing sugar is any sugar that is capable of acting as a reducing agent. In an alkaline solution, a reducing sugar forms some aldehyde or ketone, which allows it to act as a reducing agent, for example in Benedict's reagent. In such a reaction, the sugar becomes a carboxylic acid.

Barfoed's test is a chemical test used for detecting the presence of monosaccharides. It is based on the reduction of copper(II) acetate to copper(I) oxide (Cu2O), which forms a brick-red precipitate.

Potassium dichromate, K2Cr2O7, is a common inorganic chemical reagent, most commonly used as an oxidizing agent in various laboratory and industrial applications. As with all hexavalent chromium compounds, it is acutely and chronically harmful to health. It is a crystalline ionic solid with a very bright, red-orange color. The salt is popular in laboratories because it is not deliquescent, in contrast to the more industrially relevant salt sodium dichromate.

Tollens' reagent is a chemical reagent used to distinguish between aldehydes and ketones along with some alpha-hydroxy ketones which can tautomerize into aldehydes. The reagent consists of a solution of silver nitrate, ammonium hydroxide and some sodium hydroxide. It was named after its discoverer, the German chemist Bernhard Tollens. A positive test with Tollens' reagent is indicated by the precipitation of elemental silver, often producing a characteristic "silver mirror" on the inner surface of the reaction vessel.

Copper(I) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl. The substance is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. Impure samples appear green due to the presence of copper(II) chloride (CuCl2).

In chemistry, a chemical test is a qualitative or quantitative procedure designed to identify, quantify, or characterise a chemical compound or chemical group.
2,4-Dinitrophenylhydrazine (2,4-DNPH or DNPH) is the organic compound C6H3(NO2)2NHNH2. DNPH is a red to orange solid. It is a substituted hydrazine. The solid is relatively sensitive to shock and friction. For this reason DNPH is usually handled as a wet powder. DNPH is a precursor to the drug Sivifene.

The Schiff test is an early organic chemistry named reaction developed by Hugo Schiff, and is a relatively general chemical test for detection of many organic aldehydes that has also found use in the staining of biological tissues. The Schiff reagent is the reaction product of a dye formulation such as fuchsin and sodium bisulfite; pararosaniline and new fuchsin are not dye alternatives with comparable detection chemistry.

The bicinchoninic acid assay, also known as the Smith assay, after its inventor, Paul K. Smith at the Pierce Chemical Company, now part of Thermo Fisher Scientific, is a biochemical assay for determining the total concentration of protein in a solution, similar to Lowry protein assay, Bradford protein assay or biuret reagent. The total protein concentration is exhibited by a color change of the sample solution from blue to purple in proportion to protein concentration, which can then be measured using colorimetric techniques. The BCA assay was patented by Pierce Chemical Company in 1989 & the patent expired in 2006.

In chemistry, the biuret test, also known as Piotrowski's test, is a chemical test used for detecting the presence of at least two peptide bonds in a molecule. In the presence of peptides, a copper(II) ion forms mauve-colored coordination complexes in an alkaline solution. The reaction was first observed in 1833; In Poland, the biuret test is also known as Piotrowski's test in honor of the Polish physiologist Gustaw Piotrowski who independently rediscovered it in 1857. Several variants on the test have been developed, such as the BCA test and the Modified Lowry test.
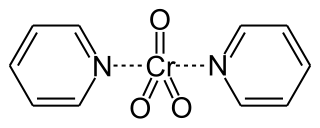
Collins reagent is the complex of chromium(VI) oxide with pyridine in dichloromethane. This metal-pyridine complex, a red solid, is used to oxidize primary alcohols to the corresponding aldehydes and secondary alcohols to the corresponding ketones. This complex is a hygroscopic orange solid.
Dextrose equivalent (DE) is a measure of the amount of reducing sugars present in a sugar product, expressed as a percentage on a dry basis relative to dextrose. The dextrose equivalent gives an indication of the average degree of polymerisation (DP) for starch sugars. As a rule of thumb, DE × DP = 120.
















