
Parvoviruses are a family of animal viruses that constitute the family Parvoviridae. They have linear, single-stranded DNA (ssDNA) genomes that typically contain two genes encoding for a replication initiator protein, called NS1, and the protein the viral capsid is made of. The coding portion of the genome is flanked by telomeres at each end that form into hairpin loops that are important during replication. Parvovirus virions are small compared to most viruses, at 23–28 nanometers in diameter, and contain the genome enclosed in an icosahedral capsid that has a rugged surface.
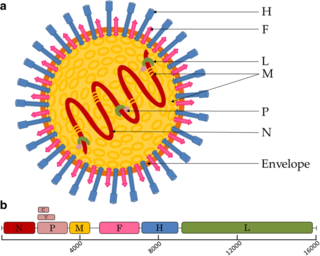
Paramyxoviridae is a family of negative-strand RNA viruses in the order Mononegavirales. Vertebrates serve as natural hosts. Diseases associated with this family include measles, mumps, and respiratory tract infections. The family has four subfamilies, 17 genera, and 78 species, three genera of which are unassigned to a subfamily.

Morbillivirus is a genus of viruses in the order Mononegavirales, in the family Paramyxoviridae. Humans, dogs, cats, cattle, seals, and cetaceans serve as natural hosts. This genus includes seven species. Diseases in humans associated with viruses classified in this genus include measles: fever, and rash; in animals, they include acute febrile respiratory tract infection.

A poliovirus, the causative agent of polio, is a serotype of the species Enterovirus C, in the family of Picornaviridae. There are three poliovirus serotypes: types 1, 2, and 3.

Hepadnaviridae is a family of viruses. Humans, apes, and birds serve as natural hosts. There are currently 18 species in this family, divided among 5 genera. Its best-known member is hepatitis B virus. Diseases associated with this family include: liver infections, such as hepatitis, hepatocellular carcinomas, and cirrhosis. It is the sole accepted family in the order Blubervirales.

Picornaviruses are a group of related nonenveloped RNA viruses which infect vertebrates including fish, mammals, and birds. They are viruses that represent a large family of small, positive-sense, single-stranded RNA viruses with a 30 nm icosahedral capsid. The viruses in this family can cause a range of diseases including the common cold, poliomyelitis, meningitis, hepatitis, and paralysis.

Papillomaviridae is a family of non-enveloped DNA viruses whose members are known as papillomaviruses. Several hundred species of papillomaviruses, traditionally referred to as "types", have been identified infecting all carefully inspected mammals, but also other vertebrates such as birds, snakes, turtles and fish. Infection by most papillomavirus types, depending on the type, is either asymptomatic or causes small benign tumors, known as papillomas or warts. Papillomas caused by some types, however, such as human papillomaviruses 16 and 18, carry a risk of becoming cancerous.
Bornaviridae is a family of negative-strand RNA viruses in the order Mononegavirales. Horses, sheep, cattle, rodents, birds, reptiles, and humans serve as natural hosts. Diseases associated with bornaviruses include Borna disease, a fatal neurologic disease of mammals restricted to central Europe; and proventricular dilatation disease (PDD) in birds. Bornaviruses may cause encephalitis in mammals like horses or sheep. The family includes 11 species assigned to three genera.

Measles morbillivirus(MeV), also called measles virus (MV), is a single-stranded, negative-sense, enveloped, non-segmented RNA virus of the genus Morbillivirus within the family Paramyxoviridae. It is the cause of measles. Humans are the natural hosts of the virus; no animal reservoirs are known to exist.

Lassa mammarenavirus (LASV) is an arenavirus that causes Lassa hemorrhagic fever, a type of viral hemorrhagic fever (VHF), in humans and other primates. Lassa mammarenavirus is an emerging virus and a select agent, requiring Biosafety Level 4-equivalent containment. It is endemic in West African countries, especially Sierra Leone, the Republic of Guinea, Nigeria, and Liberia, where the annual incidence of infection is between 300,000 and 500,000 cases, resulting in 5,000 deaths per year.

Tombusviridae is a family of single-stranded positive sense RNA plant viruses. There are three subfamilies, 17 genera, and 95 species in this family. The name is derived from Tomato bushy stunt virus (TBSV).

Viral replication is the formation of biological viruses during the infection process in the target host cells. Viruses must first get into the cell before viral replication can occur. Through the generation of abundant copies of its genome and packaging these copies, the virus continues infecting new hosts. Replication between viruses is greatly varied and depends on the type of genes involved in them. Most DNA viruses assemble in the nucleus while most RNA viruses develop solely in cytoplasm.

Gammaretrovirus is a genus in the Retroviridae family. Example species are the murine leukemia virus and the feline leukemia virus. They cause various sarcomas, leukemias and immune deficiencies in mammals, reptiles and birds.
Aquaparamyxovirus is a genus of viruses in the family Paramyxoviridae, order Mononegavirales. The genus includes two species. Fish serve as the natural hosts for AsaPV, in which the virus may cause proliferative gill inflammation.
Triatoma virus (TrV) is a virus belonging to the insect virus family Dicistroviridae. Within this family, there are currently 3 genera and 15 species of virus. Triatoma virus belongs to the genus Cripavirus. It is non-enveloped and its genetic material is positive-sense, single-stranded RNA. The natural hosts of triatoma virus are invertebrates. TrV is a known pathogen to Triatoma infestans, the major vector of Chagas disease in Argentina which makes triatoma virus a major candidate for biological vector control as opposed to chemical insecticides. Triatoma virus was first discovered in 1984 when a survey of pathogens of triatomes was conducted in the hopes of finding potential biological control methods for T. infestans.

Avian metaavulavirus 2, formerly Avian paramyxovirus 2, is a species of virus belonging to the family Paramyxoviridae and genus Metaavulavirus. The virus is a negative strand RNA virus containing a monopartite genome. Avian metaavulavirus 2 is one of nine species belonging to the genus Metaavulavirus. The most common serotype of Avulavirinae is serotype 1, the cause of Newcastle disease (ND). Avian metaavulavirus 2 has been known to cause disease, specifically mild respiratory infections in domestic poultry, including turkeys and chickens, and has many economic effects on egg production and poultry industries. The virus was first isolated from a strain in Yucaipa, California in 1956. Since then, other isolates of the virus have been isolated worldwide.

The black queen cell virus (BQCV) is a virus that infects honey bees, specifically Apis mellifera, Apis florea, and Apis dorsata. Infection of the latter two species is more recent and can be attributed to genetic similarity and geographical closeness. It is important to learn about this virus because it is one of the most common bee viruses and bees are the most important pollinators. The agricultural industry depends on the bee's pollination to increase its economic value.
Bat mumps orthorubulavirus, formerly Bat mumps rubulavirus (BMV), is a member of genus Orthorubulavirus, family Paramyxoviridae, and order Mononegavirales. Paramyxoviridae viruses were first isolated from bats using heminested PCR with degenerate primers. This process was then followed by Sanger sequencing. A specific location of this virus is not known because it was isolated from bats worldwide. Although multiple paramyxoviridae viruses have been isolated worldwide, BMV specifically has not been isolated thus far. However, BMV was detected in African fruit bats, but no infectious form has been isolated to date. It is known that BMV is transmitted through saliva in the respiratory system of bats. While the virus was considered its own species for a few years, phylogenetic analysis has since shown that it is a member of Mumps orthorubulavirus.

Astroviridae is a family of non-enveloped ssRNA viruses that cause infections in different animals. The family name is derived from the Greek word astron ("star") referring to the star-like appearance of spikes projecting from the surface of these small unenveloped viruses. Astroviruses were initially identified in humans but have since been isolated from other mammals and birds. This family of viruses consists of two genera, Avastrovirus (AAstV) and Mamastrovirus (MAstV). Astroviruses most frequently cause infection of the gastrointestinal tract but in some animals they may result in encephalitis, hepatitis (avian) and nephritis (avian).
Rio Negro virus is an alphavirus that was first isolated in Argentina in 1980. The virus was first called Ag80-663 but was renamed to Rio Negro virus in 2005. It is a former member of the Venezuelan equine encephalitis complex (VEEC), which are a group of alphaviruses in the Americas that have the potential to emerge and cause disease. Río Negro virus was recently reclassified as a distinct species. Closely related viruses include Mucambo virus and Everglades virus.














