| Morbillivirus | |
|---|---|
 | |
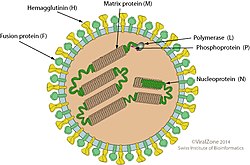
| Measles virus electron micrograph | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Riboviria |
| Kingdom: | Orthornavirae |
| Phylum: | Negarnaviricota |
| Class: | Monjiviricetes |
| Order: | Mononegavirales |
| Family: | Paramyxoviridae |
| Subfamily: | Orthoparamyxovirinae |
| Genus: | Morbillivirus |
| Species | |
Morbillivirus is a genus of viruses in the order Mononegavirales , in the family Paramyxoviridae . [1] [2] Humans, dogs, cats, cattle, seals, and cetaceans serve as natural hosts. This genus contains 10 species, one of which is extinct. Diseases in humans associated with viruses classified in this genus include measles; in animals, they include acute febrile respiratory tract infection and Canine distemper. [3] In 2013, a wave of increased death among the Common bottlenose dolphin population was attributed to morbillivirus. [4]