
A-DNA is one of the possible double helical structures which DNA can adopt. A-DNA is thought to be one of three biologically active double helical structures along with B-DNA and Z-DNA. It is a right-handed double helix fairly similar to the more common B-DNA form, but with a shorter, more compact helical structure whose base pairs are not perpendicular to the helix-axis as in B-DNA. It was discovered by Rosalind Franklin, who also named the A and B forms. She showed that DNA is driven into the A form when under dehydrating conditions. Such conditions are commonly used to form crystals, and many DNA crystal structures are in the A form. The same helical conformation occurs in double-stranded RNAs, and in DNA-RNA hybrid double helices.
Icerudivirus is a genus of viruses in the family Rudiviridae. These viruses are non-enveloped, stiff-rod-shaped viruses with linear dsDNA genomes, that infect hyperthermophilic archaea of the species Sulfolobus islandicus. There are three species in the genus.

Lipothrixviridae is a family of viruses in the order Ligamenvirales. Thermophilic archaea in the phylum Thermoproteota serve as natural hosts. There are 11 species in this family, assigned to 4 genera.
Fuselloviridae is a family of viruses. Sulfolobus species, specifically shibatae, solfataricus, and islandicus, serve as natural hosts. There are two genera and nine species in the family. The Fuselloviridae are ubiquitous in high-temperature (≥70 °C), acidic hot springs around the world.
Guttaviridae is a family of viruses. Archaea serve as natural hosts. There are two genera in this family, containing one species each. The name is derived from the Latin gutta, meaning 'droplet'.

Globuloviridae is a family of hyperthermophilic archaeal viruses. Crenarchaea of the genera Pyrobaculum and Thermoproteus serve as natural hosts. There are four species in this family, assigned to a single genus, Alphaglobulovirus.

Bottigliavirus is the only genus in the family Ampullaviridae and contains 3 species. Ampullaviridae infect archaea of the genus Acidianus. The name of the family and genus is derived from the Latin word for bottle, ampulla, due to the virions having the shape of a bottle. The family was first described during an investigation of the microbial flora of hot springs in Italy.

Bicaudaviridae is a family of hyperthermophilic archaeal viruses. Members of the genus Acidianus serve as natural hosts. There is only one genus, Bicaudavirus, and one species, Acidianus two-tailed virus, in this family. However, Sulfolobus tengchongensis spindle-shaped viruses 1 and 2 are regarded to belong to this family also.

Clavaviridae is a family of double-stranded viruses that infect archaea. This family was first described by the team led by D. Prangishvili in 2010. There is one genus in this family (Clavavirus). Within this genus, a single species has been described to date: Aeropyrum pernix bacilliform virus 1 (APBV1).
Ligamenvirales is an order of linear viruses that infect archaea of the phylum Thermoproteota and have double-stranded DNA genomes. The order was proposed by David Prangishvili and Mart Krupovic in 2012 and subsequently created by the International Committee on Taxonomy of Viruses (ICTV).

David Prangishvili is a virologist, Professor at the Pasteur Institute of Paris, and foremost authority on viruses infecting Archaea.
Yingchengvirus is a genus of double stranded DNA viruses that infect haloarchaea. The genus was previously named Betasphaerolipovirus.
Alphafusellovirus is a genus of viruses, in the family Fuselloviridae. Species in the genus Sulfolobus serve as natural hosts. There are seven species in this genus.
Spiraviridae is a family of incertae sedis viruses that replicate in hyperthermophilic archaea of the genus Aeropyrum, specifically Aeropyrum pernix. The family contains one genus, Alphaspiravirus, which contains one species, Aeropyrum coil-shaped virus. The virions of ACV are non-enveloped and in the shape of hollow cylinders that are formed by a coiling fiber that consists of two intertwining halves of the circular DNA strand inside a capsid. An appendage protrudes from each end of the cylindrical virion. The viral genome is ssDNA(+) and encodes for significantly more genes than other known ssDNA viruses. ACV is also unique in that it appears to lack its own enzymes to aid replication, instead likely using the host cell's replisomes. ACV has no known relation to any other archaea-infecting viruses, but it does share its coil-like morphology with some other archaeal viruses, suggesting that such viruses may be an ancient lineage that only infect archaea.
Sulfolobus islandicus rod-shaped virus 2, also referred to as SIRV2, is an archaeal virus whose only known host is the archaeon Sulfolobus islandicus. This virus belongs to the family Rudiviridae. Like other viruses in the family, it is common in geothermal environments.
Sulfolobus islandicus filamentous virus (SIFV) is an archaeal virus, classified in the family Lipothrixviridae within the order Ligamenvirales. The virus infects hypethermophilic and acidophilic archaeon Sulfolobus islandicus.
In virology, realm is the highest taxonomic rank established for viruses by the International Committee on Taxonomy of Viruses (ICTV), which oversees virus taxonomy. Six virus realms are recognized and united by specific highly conserved traits:

An archaeal virus is a virus that infects and replicates in archaea, a domain of unicellular, prokaryotic organisms. Archaeal viruses, like their hosts, are found worldwide, including in extreme environments inhospitable to most life such as acidic hot springs, highly saline bodies of water, and at the bottom of the ocean. They have been also found in the human body. The first known archaeal virus was described in 1974 and since then, a large diversity of archaeal viruses have been discovered, many possessing unique characteristics not found in other viruses. Little is known about their biological processes, such as how they replicate, but they are believed to have many independent origins, some of which likely predate the last archaeal common ancestor (LACA).
Portogloboviridae is a family of dsDNA viruses that infect archaea. It is a proposed family of the realm Varidnaviria, but ICTV officially puts it as incertae sedis virus. Viruses in the family are related to Helvetiavirae. The capsid proteins of these viruses and their characteristics are of evolutionary importance for the origin of the other Varidnaviria viruses since they seem to retain primordial characters.

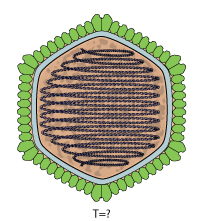
Adnaviria is a realm of viruses that includes archaeal viruses that have a filamentous virion and a linear, double-stranded DNA genome. The genome exists in A-form (A-DNA) and encodes a dimeric major capsid protein (MCP) that contains the SIRV2 fold, a type of alpha-helix bundle containing four helices. The virion consists of the genome encased in capsid proteins to form a helical nucleoprotein complex. For some viruses, this helix is surrounded by a lipid membrane called an envelope. Some contain an additional protein layer between the nucleoprotein helix and the envelope. Complete virions are long and thin and may be flexible or a stiff like a rod.








