A retrovirus is a type of virus that inserts a DNA copy of its RNA genome into the DNA of a host cell that it invades, thus changing the genome of that cell. After invading a host cell's cytoplasm, the virus uses its own reverse transcriptase enzyme to produce DNA from its RNA genome, the reverse of the usual pattern, thus retro (backwards). The new DNA is then incorporated into the host cell genome by an integrase enzyme, at which point the retroviral DNA is referred to as a provirus. The host cell then treats the viral DNA as part of its own genome, transcribing and translating the viral genes along with the cell's own genes, producing the proteins required to assemble new copies of the virus. Many retroviruses cause serious diseases in humans, other mammals, and birds.
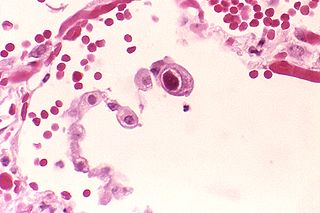
Cytomegalovirus (CMV) is a genus of viruses in the order Herpesvirales, in the family Herpesviridae, in the subfamily Betaherpesvirinae. Humans and other primates serve as natural hosts. The 11 species in this genus include human betaherpesvirus 5, which is the species that infects humans. Diseases associated with HHV-5 include mononucleosis and pneumonia, and congenital CMV in infants can lead to deafness and ambulatory problems.
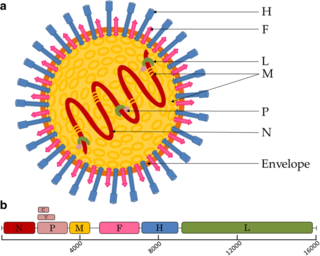
Paramyxoviridae is a family of negative-strand RNA viruses in the order Mononegavirales. Vertebrates serve as natural hosts. Diseases associated with this family include measles, mumps, and respiratory tract infections. The family has four subfamilies, 17 genera, and 78 species, three genera of which are unassigned to a subfamily.
Rhadinovirus is a genus of viruses in the order Herpesvirales, in the family Herpesviridae, in the subfamily Gammaherpesvirinae. Humans and other mammals serve as natural hosts. There are 12 species in this genus. Diseases associated with this genus include: Kaposi's sarcoma, primary effusion lymphoma and multicentric Castleman's disease, caused by Human gammaherpesvirus 8 (HHV-8), also known as Kaposi's sarcoma-associated herpesvirus (KSHV). The term rhadino comes from the Latin fragile, referring to the tendency of the viral genome to break apart when it is isolated.

Kaposi's sarcoma-associated herpesvirus (KSHV) is the ninth known human herpesvirus; its formal name according to the International Committee on Taxonomy of Viruses (ICTV) is Human gammaherpesvirus 8, or HHV-8 in short. Like other herpesviruses, its informal names are used interchangeably with its formal ICTV name. This virus causes Kaposi's sarcoma, a cancer commonly occurring in AIDS patients, as well as primary effusion lymphoma, HHV-8-associated multicentric Castleman's disease and KSHV inflammatory cytokine syndrome. It is one of seven currently known human cancer viruses, or oncoviruses. Even after many years since the discovery of KSHV/HHV8, there is no known cure for KSHV associated tumorigenesis.

Human herpesvirus 6 (HHV-6) is the common collective name for human betaherpesvirus 6A (HHV-6A) and human betaherpesvirus 6B (HHV-6B). These closely related viruses are two of the nine known herpesviruses that have humans as their primary host.

Herpesviridae is a large family of DNA viruses that cause infections and certain diseases in animals, including humans. The members of this family are also known as herpesviruses. The family name is derived from the Greek word ἕρπειν, referring to spreading cutaneous lesions, usually involving blisters, seen in flares of herpes simplex 1, herpes simplex 2 and herpes zoster (shingles). In 1971, the International Committee on the Taxonomy of Viruses (ICTV) established Herpesvirus as a genus with 23 viruses among four groups. As of 2020, 115 species are recognized, all but one of which are in one of the three subfamilies. Herpesviruses can cause both latent and lytic infections.

Herpes simplex virus1 and 2, also known by their taxonomic names Human alphaherpesvirus 1 and Human alphaherpesvirus 2, are two members of the human Herpesviridae family, a set of viruses that produce viral infections in the majority of humans. Both HSV-1 and HSV-2 are very common and contagious. They can be spread when an infected person begins shedding the virus.

Duck plague is a worldwide disease caused by Anatid alphaherpesvirus 1 (AnHV-1) of the family Herpesviridae that causes acute disease with high mortality rates in flocks of ducks, geese, and swans. It is spread both vertically and horizontally—through contaminated water and direct contact. Migratory waterfowl are a major factor in the spread of this disease as they are often asymptomatic carriers of disease. The incubation period is three to seven days. Birds as young as one week old can be infected. DEV is not zoonotic.

Gammaherpesvirinae is a subfamily of viruses in the order Herpesvirales and in the family Herpesviridae. Viruses in Gammaherpesvirinae are distinguished by reproducing at a more variable rate than other subfamilies of Herpesviridae. Mammals serve as natural hosts. There are 43 species in this subfamily, divided among 7 genera with three species unassigned to a genus. Diseases associated with this subfamily include: HHV-4: infectious mononucleosis. HHV-8: Kaposi's sarcoma.

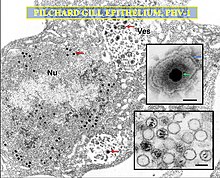
Cyvirus cyprinidallo3, also known as Cyprinid herpesvirus 3 (CyHV-3) is a species in the Genus Cyvirus and the order Herpesvirales. It causes a viral disease that is very contagious to the common carp Cyprinus carpio.

The Herpesvirales is an order of dsDNA viruses with animal hosts, characterised by a common morphology consisting of an icosahedral capsid enclosed in a glycoprotein-containing lipid envelope. Common infections in humans caused by members of this order include cold sores, genital herpes, chickenpox, shingles, and glandular fever. Herpesvirales is the sole order in the class Herviviricetes, which is the sole class in the phylum Peploviricota.
Malacoherpesviridae is a family of DNA viruses in the order Herpesvirales. Molluscs serve as natural hosts, making members of this family the only known herpesviruses to infect invertebrates. There are currently only two species recognised in this family, both classified into separate genera. Disease associated with this family includes sporadic episodes of high mortality among larvae and juveniles. The family name Malacoherpesviridae is derived from Greek word 'μαλακός (malacos) meaning 'soft' and from Greek word 'μαλάκιον (malakion) meaning 'mollusc'.
Proboscivirus is a genus of viruses in the order Herpesvirales, in the family Herpesviridae, in the subfamily Betaherpesvirinae. Elephants serve as natural hosts. EEHV1 is apathogenic for African elephants but causes fatal haemorrhagic disease in Asian elephants. The name "Proboscivirus" comes from the Greek word προβοσκίς or "proboscis" meaning "the elephant trunk," for which the virus accordingly uses as its means of contraction and transmission to enter the elephant's body.
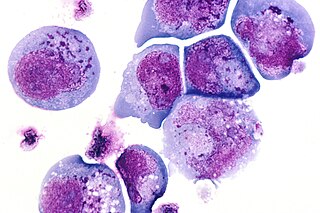
Human herpes viruses, also known as HHVs, are part of a family of DNA viruses that cause several diseases in humans. One of the most notable functions of this virus family is their ability to enter a latent phase and lay dormant within animals for extended periods of time. The mechanism that controls this is very complex because expression of viral proteins during latency is decreased a great deal, meaning that the virus must have transcription of its genes repressed. There are many factors and mechanisms that control this process and epigenetics is one way this is accomplished. Epigenetics refers to persistent changes in expression patterns that are not caused by changes to the DNA sequence. This happens through mechanisms such as methylation and acetylation of histones, DNA methylation, and non-coding RNAs (ncRNA). Altering the acetylation of histones creates changes in expression by changing the binding affinity of histones to DNA, making it harder or easier for transcription machinery to access the DNA. Methyl and acetyl groups can also act as binding sites for transcription factors and enzymes that further modify histones or alter the DNA itself.
Ictalurivirus is a genus of viruses in the order Herpesvirales, in the family Alloherpesviridae. Fish serve as natural hosts. There are three species in this genus. Diseases associated with this genus include: channel catfish disease.
Ostreavirus is a genus of viruses in the order Herpesvirales, and one of only two genera in the family Malacoherpesviridae. Molluscs serve as natural hosts. There is only one species described in this genus, Ostreid herpesvirus 1 (OsHV-1), commonly known as oyster herpesvirus. A disease associated with this genus is sporadic episodes of high mortality among larvae and juveniles.
Aurivirus is a genus of viruses in the order Herpesvirales, and one of only two genera the family Malacoherpesviridae. Haliotid molluscs serve as natural hosts. There is only one species described in this genus, Haliotid herpesvirus 1 (AbHV-1), commonly known as abalone herpesvirus. A disease associated with this virus is acute ganglioneuritis.
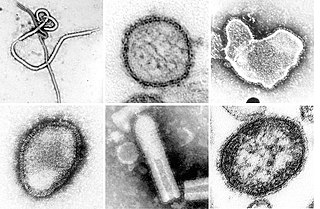
Negative-strand RNA viruses are a group of related viruses that have negative-sense, single-stranded genomes made of ribonucleic acid (RNA). They have genomes that act as complementary strands from which messenger RNA (mRNA) is synthesized by the viral enzyme RNA-dependent RNA polymerase (RdRp). During replication of the viral genome, RdRp synthesizes a positive-sense antigenome that it uses as a template to create genomic negative-sense RNA. Negative-strand RNA viruses also share a number of other characteristics: most contain a viral envelope that surrounds the capsid, which encases the viral genome, −ssRNA virus genomes are usually linear, and it is common for their genome to be segmented.
Batravirus ranidallo1, also known as Ranid herpesvirus 1 (RaHV-1), is a double-stranded DNA virus within the order Herpesvirales. The virus was initially observed within renal tumors in 1934 by Baldwin Lucké, and more recently has become identifiable through the use of PCR in samples isolated from frog tumors. RaHV-1 causes renal tumors within the northern leopard frog, Rana pipiens. The virus has not yet been isolated in vitro within cell lines, meaning that while its existence and symptoms are fairly evident, its methods of transmission, cell infection, and reproduction are largely unknown.