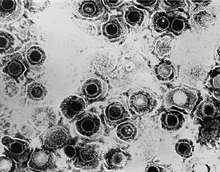
Varicella zoster virus (VZV), also known as human herpesvirus 3 or Human alphaherpesvirus 3 (taxonomically), is one of nine known herpes viruses that can infect humans. It causes chickenpox (varicella) commonly affecting children and young adults, and shingles in adults but rarely in children. As a late complication of VZV infection, Ramsay Hunt syndrome type 2 may develop in rare cases. VZV infections are species-specific to humans. The virus can survive in external environments for a few hours.

Valaciclovir, also spelled valacyclovir, is an antiviral medication used to treat outbreaks of herpes simplex or herpes zoster (shingles). It is also used to prevent cytomegalovirus following a kidney transplant in high risk cases. It is taken by mouth.

Gingivostomatitis is a combination of gingivitis and stomatitis, or an inflammation of the oral mucosa and gingiva. Herpetic gingivostomatitis is often the initial presentation during the first ("primary") herpes simplex infection. It is of greater severity than herpes labialis which is often the subsequent presentations. Primary herpetic gingivostomatitis is the most common viral infection of the mouth.

Famciclovir is a guanosine analogue antiviral drug used for the treatment of various herpesvirus infections, most commonly for herpes zoster (shingles). It is a prodrug form of penciclovir with improved oral bioavailability. Famciclovir is marketed under the trade name Famvir (Novartis).
Bovine alphaherpesvirus 1 (BoHV-1) is a virus of the family Herpesviridae and the subfamily Alphaherpesvirinae, known to cause several diseases worldwide in cattle, including rhinotracheitis, vaginitis, balanoposthitis, abortion, conjunctivitis, and enteritis. BoHV-1 is also a contributing factor in shipping fever, also known as bovine respiratory disease (BRD). It is spread horizontally through sexual contact, artificial insemination, and aerosol transmission and it may also be transmitted vertically across the placenta. BoHV-1 can cause both clinical and subclinical infections, depending on the virulence of the strain. Although these symptoms are mainly non-life-threatening it is an economically important disease as infection may cause a drop in production and affect trade restrictions. Like other herpesviruses, BoHV-1 causes a lifelong latent infection and sporadic shedding of the virus. The sciatic nerve and trigeminal nerve are the sites of latency. A reactivated latent carrier is normally the source of infection in a herd. The clinical signs displayed are dependent on the virulence of the strain. There is a vaccine available which reduces the severity and incidence of disease. Some countries in Europe have successfully eradicated the disease by applying a strict culling policy.

Herpes simplex virus1 and 2, also known by their taxonomic names Human alphaherpesvirus 1 and Human alphaherpesvirus 2, are two members of the human Herpesviridae family, a set of viruses that produce viral infections in the majority of humans. Both HSV-1 and HSV-2 are very common and contagious. They can be spread when an infected person begins shedding the virus.

Genital herpes is a herpes infection of the genitals caused by the herpes simplex virus (HSV). Most people either have no or mild symptoms and thus do not know they are infected. When symptoms do occur, they typically include small blisters that break open to form painful ulcers. Flu-like symptoms, such as fever, aching, or swollen lymph nodes, may also occur. Onset is typically around 4 days after exposure with symptoms lasting up to 4 weeks. Once infected further outbreaks may occur but are generally milder.

Duck plague is a worldwide disease caused by Anatid alphaherpesvirus 1 (AnHV-1) of the family Herpesviridae that causes acute disease with high mortality rates in flocks of ducks, geese, and swans. It is spread both vertically and horizontally—through contaminated water and direct contact. Migratory waterfowl are a major factor in the spread of this disease as they are often asymptomatic carriers of disease. The incubation period is three to seven days. Birds as young as one week old can be infected. DEV is not zoonotic.
Herpes gladiatorum is one of the most infectious of herpes-caused diseases, and is transmissible by skin-to-skin contact. The disease was first described in the 1960s in the New England Journal of Medicine. It is caused by contagious infection with human herpes simplex virus type 1 (HSV-1), which more commonly causes oral herpes. Another strain, HSV-2 usually causes genital herpes, although the strains are very similar and either can cause herpes in any location.
Lawrence Corey is an American immunologist and virologist known for his work in the development of antiviral therapies and vaccines, particularly for herpes simplex virus (HSV) and HIV/AIDS.

Herpes simplex, often known simply as herpes, is a viral infection caused by the herpes simplex virus. Herpes infections are categorized by the area of the body that is infected. The two major types of herpes are oral herpes and genital herpes, though other forms also exist.
Neonatal herpes simplex, or simply neonatal herpes, is a herpes infection in a newborn baby, caused by the herpes simplex virus (HSV). It occurs mostly as a result of vertical transmission of the HSV from an affected mother to her baby. Types include skin, eye, and mouth herpes (SEM), disseminated herpes (DIS), and central nervous system herpes (CNS). Depending on the type, symptoms vary from a fever to small blisters, irritability, low body temperature, lethargy, breathing difficulty, and a large abdomen due to ascites or large liver. There may be red streaming eyes or no symptoms.
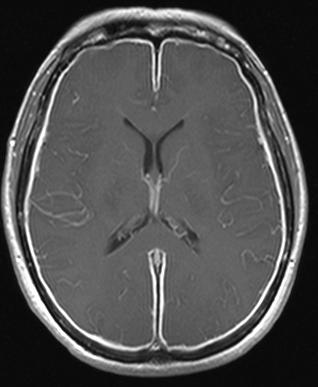
Herpes meningitis is inflammation of the meninges, the protective tissues surrounding the spinal cord and brain, due to infection from viruses of the Herpesviridae family - the most common amongst adults is HSV-2. Symptoms are self-limiting over 2 weeks with severe headache, nausea, vomiting, neck-stiffness, and photophobia. Herpes meningitis can cause Mollaret's meningitis, a form of recurrent meningitis. Lumbar puncture with cerebrospinal fluid results demonstrating aseptic meningitis pattern is necessary for diagnosis and polymerase chain reaction is used to detect viral presence. Although symptoms are self-limiting, treatment with antiviral medication may be recommended to prevent progression to Herpes Meningoencephalitis.

Herpes simplex encephalitis (HSE), or simply herpes encephalitis, is encephalitis due to herpes simplex virus. It is estimated to affect at least 1 in 500,000 individuals per year, and some studies suggest an incidence rate of 5.9 cases per 100,000 live births.

A cold sore is a type of herpes infection caused by the herpes simplex virus that affects primarily the lip. Symptoms typically include a burning pain followed by small blisters or sores. The first attack may also be accompanied by fever, sore throat, and enlarged lymph nodes. The rash usually heals within ten days, but the virus remains dormant in the trigeminal ganglion. The virus may periodically reactivate to create another outbreak of sores in the mouth or lip.
The epidemiology of herpes simplex is of substantial epidemiologic and public health interest. Worldwide, the rate of infection with herpes simplex virus—counting both HSV-1 and HSV-2—is around 90%. Although many people infected with HSV develop labial or genital lesions, the majority are either undiagnosed or display no physical symptoms—individuals with no symptoms are described as asymptomatic or as having subclinical herpes.
Herpes simplex research includes all medical research that attempts to prevent, treat, or cure herpes, as well as fundamental research about the nature of herpes. Examples of particular herpes research include drug development, vaccines and genome editing. HSV-1 and HSV-2 are commonly thought of as oral and genital herpes respectively, but other members in the herpes family include chickenpox (varicella/zoster), cytomegalovirus, and Epstein-Barr virus. There are many more virus members that infect animals other than humans, some of which cause disease in companion animals or have economic impacts in the agriculture industry.

Pritelivir is a direct-acting antiviral drug in development for the treatment of herpes simplex virus infections (HSV). This is particularly important in immune compromised patients. It is currently in Phase III clinical development by the German biopharmaceutical company AiCuris Anti-infective Cures AG. US FDA granted fast track designation for pritelivir in 2017 and breakthrough therapy designation 2020.
HSV epigenetics is the epigenetic modification of herpes simplex virus (HSV) genetic code.

Human alphaherpesvirus 1 or Herpes simplex virus 1 is a species of virus in the genus Simplexvirus, subfamily Alphaherpesvirinae, family Herpesviridae, and order Herpesvirales.