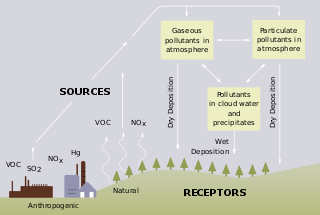
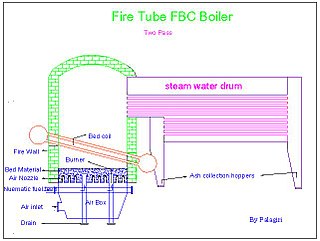
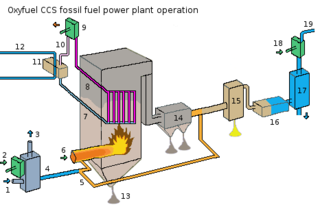
Flue-gas emissions from fossil-fuel combustion refers to the combustion-product gas resulting from the burning of fossil fuels. [1] Most fossil fuels are combusted with ambient air (as differentiated from combustion with pure oxygen). Since ambient air contains about 79 volume percent gaseous nitrogen (N2), [2] which is essentially non-combustible, the largest part of the flue gas from most fossil-fuel combustion is uncombusted nitrogen. Carbon dioxide (CO2), the next largest part of flue gas, can be as much as 10−25 volume percent or more of the flue gas. This is closely followed in volume by water vapor (H2O) created by the combustion of the hydrogen in the fuel with atmospheric oxygen. Much of the 'smoke' seen pouring from flue gas stacks is this water vapor forming a cloud as it contacts cool air.

Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion in a fire produces a flame, and the heat produced can make combustion self-sustaining. Combustion is often a complicated sequence of elementary radical reactions. Solid fuels, such as wood and coal, first undergo endothermic pyrolysis to produce gaseous fuels whose combustion then supplies the heat required to produce more of them. Combustion is often hot enough that incandescent light in the form of either glowing or a flame is produced. A simple example can be seen in the combustion of hydrogen and oxygen into water vapor, a reaction commonly used to fuel rocket engines. This reaction releases 242 kJ/mol of heat and reduces the enthalpy accordingly :

A fossil fuel is a fuel formed by natural processes, such as anaerobic decomposition of buried dead organisms, containing energy originating in ancient photosynthesis. The age of the organisms and their resulting fossil fuels is typically millions of years, and sometimes exceeds 650 million years. Fossil fuels contain high percentages of carbon and include petroleum, coal, and natural gas. Other commonly used derivatives include kerosene and propane. Fossil fuels range from volatile materials with low carbon to hydrogen ratios like methane, to liquids like petroleum, to nonvolatile materials composed of almost pure carbon, like anthracite coal. Methane can be found in hydrocarbon fields either alone, associated with oil, or in the form of methane clathrates.

Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group on the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. By mass, oxygen is the third-most abundant element in the universe, after hydrogen and helium. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula O
2. Diatomic oxygen gas constitutes 20.8% of the Earth's atmosphere. As compounds including oxides, the element makes up almost half of the Earth's crust.
Contents
A typical flue gas from the combustion of fossil fuels contains very small amounts of nitrogen oxides (NOx), sulfur dioxide (SO2) and particulate matter. [1] The nitrogen oxides are derived from the nitrogen in the ambient air as well as from any nitrogen-containing compounds in the fossil fuel. The sulfur dioxide is derived from any sulfur-containing compounds in the fuels. The particulate matter is composed of very small particles of solid materials and very small liquid droplets which give flue gases their smoky appearance.
Nitrogen oxide may refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds:

Sulfur dioxide is the chemical compound with the formula SO
2. It is a toxic gas with a burnt match smell. It is released naturally by volcanic activity and is produced as a by-product of the burning of fossil fuels contaminated with sulfur compounds and copper extraction.
The steam generators in large power plants and the process furnaces in large refineries, petrochemical and chemical plants, and incinerators burn considerable amounts of fossil fuels and therefore emit large amounts of flue gas to the ambient atmosphere. The table below presents the total amounts of flue gas typically generated by the burning of fossil fuels such as natural gas, fuel oil and coal. The data were obtained by stoichiometric [3] calculations. [4]

A furnace is a device used for high-temperature heating. The name derives from Latin word fornax, which means oven. The heat energy to fuel a furnace may be supplied directly by fuel combustion, by electricity such as the electric arc furnace, or through induction heating in induction furnaces.

Oil refinery or petroleum refinery is an industrial process plant where crude oil is transformed and refined into more useful products such as petroleum naphtha, gasoline, diesel fuel, asphalt base, heating oil, kerosene, liquefied petroleum gas, jet fuel and fuel oils. Petrochemicals feed stock like ethylene and propylene can also be produced directly by cracking crude oil without the need of using refined products of crude oil such as naphtha.

Petrochemicals are chemical products derived from petroleum. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as corn, palm fruit or sugar cane.
It is of interest to note that the total amount of wet flue gas generated by coal combustion is only 10 percent higher than the flue gas generated by natural-gas combustion (the ratio for dry flue gas is higher).
| Combustion data | Fuel gas | Fuel oil | Coal |
|---|---|---|---|
| Fuel properties: | |||
| Gross caloric value, MJ/m3 | 43.01 | ||
| Gross heating value, Btu/scf | 1,093 | ||
| Gross caloric value, MJ/kg | 43.50 | ||
| Gross heating value, Btu/gal[ vague ] | 150,000 | ||
| Gross caloric value, MJ/kg | 25.92 | ||
| Gross heating value, Btu/lb | 11,150 | ||
| Molecular weight | 18 | ||
| Specific gravity | 0.9626 | ||
| Gravity, °API | 15.5 | ||
| Carbon/hydrogen ratio by weight | 8.1 | ||
| weight % carbon | 61.2 | ||
| weight % hydrogen | 4.3 | ||
| weight % oxygen | 7.4 | ||
| weight % sulfur | 3.9 | ||
| weight % nitrogen | 1.2 | ||
| weight % ash | 12.0 | ||
| weight % moisture | 10.0 | ||
| Combustion air: | |||
| Excess combustion air, % | 12 | 15 | 20 |
| Wet exhaust flue gas: | |||
| Amount of wet exhaust gas, m3/GJ of fuel | 294.8 | 303.1 | 323.1 |
| Amount of wet exhaust gas, scf/106 Btu of fuel | 11,600 | 11,930 | 12,714 |
| CO2 in wet exhaust gas, volume % | 8.8 | 12.4 | 13.7 |
| O2 in wet exhaust gas, volume % | 2.0 | 2.6 | 3.4 |
| Molecular weight of wet exhaust gas | 27.7 | 29.0 | 29.5 |
| Dry exhaust flue gas: | |||
| Amount of dry exhaust gas, m3/GJ of fuel | 241.6 | 269.3 | 293.6 |
| Amount of dry exhaust gas, scf/106 Btu of fuel | 9,510 | 10,600 | 11,554 |
| CO2 in dry exhaust gas, volume % | 10.8 | 14.0 | 15.0 |
| O2 in dry exhaust gas, volume % | 2.5 | 2.9 | 3.7 |
| Molecular weight of dry exhaust gas | 29.9 | 30.4 | 30.7 |
- Note: m3 are standard cubic meters at 0 °C and 101.325 kPa, and scf is standard cubic feet at 60 °F and 14.696 psia.