
In organic chemistry, an oxime is an organic compound belonging to the imines, with the general formula RR’C=N−OH, where R is an organic side-chain and R' may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds. Amidoximes are oximes of amides with general structure R1C(=NOH)NR2R3.

Iodoform is the organoiodine compound with the chemical formula CHI3. It is a pale yellow, crystalline, volatile substance, with a penetrating and distinctive odor and, analogous to chloroform, sweetish taste. It is occasionally used as a disinfectant.
Chromic acid is an inorganic acid composed of the elements chromium, oxygen, and hydrogen. It is a dark, purplish red, odorless, sand-like solid powder. When dissolved in water, it is a strong acid. There are 2 types of chromic acid: molecular chromic acid with the formula H
2CrO
4 and dichromic acid with the formula H
2Cr
2O
7.
Methyl ethyl ketone peroxide (MEKP) is an organic peroxide with the formula [(CH3)(C2H5)C(O2H)]2O2. MEKP is a colorless oily liquid. It is widely used in vulcanization (crosslinking) of polymers.
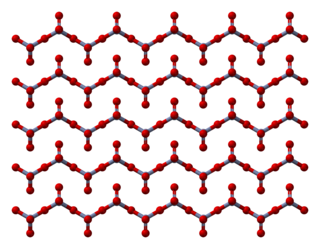
Chromium trioxide (also known as chromium(VI) oxide or chromic anhydride) is an inorganic compound with the formula CrO3. It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name. This compound is a dark-purple solid under anhydrous conditions and bright orange when wet. The substance dissolves in water concomitant with hydrolysis. Millions of kilograms are produced annually, mainly for electroplating. Chromium trioxide is a powerful oxidiser, a mutagen, and a carcinogen.

In organic chemistry, organic peroxides are organic compounds containing the peroxide functional group. If the R′ is hydrogen, the compounds are called hydroperoxides, which are discussed in that article. The O−O bond of peroxides easily breaks, producing free radicals of the form RO•. Thus, organic peroxides are useful as initiators for some types of polymerization, such as the acrylic, unsaturated polyester, and vinyl ester resins used in glass-reinforced plastics. MEKP and benzoyl peroxide are commonly used for this purpose. However, the same property also means that organic peroxides can explosively combust. Organic peroxides, like their inorganic counterparts, are often powerful bleaching agents.

Phenylacetone, also known as phenyl-2-propanone, is an organic compound with the chemical formula C6H5CH2COCH3. It is a colorless oil that is soluble in organic solvents. It is a mono-substituted benzene derivative, consisting of an acetone attached to a phenyl group. As such, its systematic IUPAC name is 1-phenyl-2-propanone.

Bromoacetone is an organic compound with the formula CH3COCH2Br. It is a colorless liquid although impure samples appear yellow or even brown. It is a lachrymatory agent and a precursor to other organic compounds.
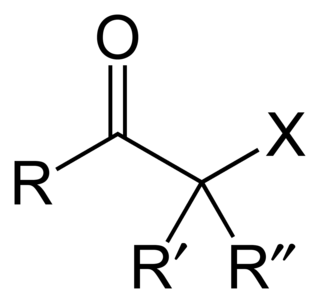
In organic chemistry, an α-halo ketone is a functional group consisting of a ketone group or more generally a carbonyl group with an α-halogen substituent. α-Halo ketones are alkylating agents. Prominent α-halo ketones include phenacyl bromide and chloroacetone.

The Stork enamine alkylation involves the addition of an enamine to a Michael acceptor or another electrophilic alkylation reagent to give an alkylated iminium product, which is hydrolyzed by dilute aqueous acid to give the alkylated ketone or aldehyde. Since enamines are generally produced from ketones or aldehydes, this overall process constitutes a selective monoalkylation of a ketone or aldehyde, a process that may be difficult to achieve directly.
White Cross (Weiẞkreuz) is a World War I chemical warfare agent consisting of one or more lachrymatory agents: bromoacetone (BA), bromobenzyl cyanide (Camite), bromomethyl ethyl ketone, chloroacetone, ethyl bromoacetate, and/or xylyl bromide.
Ammonium formate, NH4HCO2, is the ammonium salt of formic acid. It is a colorless, hygroscopic, crystalline solid.

Chloroacetone is a chemical compound with the formula CH3COCH2Cl. At STP it is a colourless liquid with a pungent odour. On exposure to light, it turns to a dark yellow-amber colour. It was used as a tear gas in World War I.

Dioxane tetraketone (or 1,4-dioxane-2,3,5,6-tetrone) is an organic compound with the formula C4O6. It is an oxide of carbon (an oxocarbon), which can be viewed as the fourfold ketone of dioxane. It can also be viewed as the cyclic dimer of oxiranedione (C2O3), the hypothetical anhydride of oxalic acid.

Bis(chloromethyl) ketone is a chemical substance with formula C
3H
4Cl
2O. It is a solid, and is used in the making of citric acid. Exposures such as contact or inhalation of bis(chloromethyl) ketone can result in irritation or damage to skin, eyes, throat, lungs, liver and kidneys, as well as headaches and fainting.

Alpha-substitution reactions occur at the position next to the carbonyl group, the α-position, and involve the substitution of an α hydrogen atom by an electrophile, E, through either an enol or enolate ion intermediate.

βk-2C-B is a novel psychedelic substance. It is the beta (β) ketone structural analogue of 2C-B, a psychedelic drug of the 2C family. It is used as a recreational drug, usually taken orally. βk-2C-B is a controlled substance in Canada, Germany, Switzerland, and the United Kingdom.

Thioacetone is an organosulfur compound belonging to the -thione group called thioketones with a chemical formula (CH3)2CS. It is an unstable orange or brown substance that can be isolated only at low temperatures. Above −20 °C (−4 °F), thioacetone readily converts to a polymer and a trimer, trithioacetone. It has an extremely potent, unpleasant odor, and is considered one of the worst-smelling chemicals known to humanity.
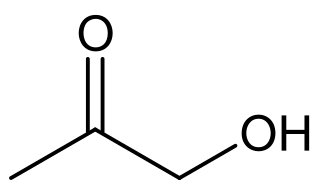
Hydroxyacetone, also known as acetol, is the organic chemical with the formula CH3C(O)CH2OH. It consists of a primary alcohol substituent on acetone. It is an α-hydroxyketone, also called a ketol, and is the simplest hydroxy ketone structure. It is a colorless, distillable liquid.

Iodoacetone is an organoiodine compound with the chemical formula C
3H
5IO The substance is a colorless liquid under normal conditions, soluble in ethanol.



















