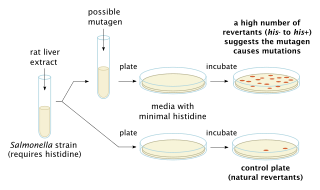
The Ames test is a widely employed method that uses bacteria to test whether a given chemical can cause mutations in the DNA of the test organism. More formally, it is a biological assay to assess the mutagenic potential of chemical compounds. A positive test indicates that the chemical is mutagenic and therefore may act as a carcinogen, because cancer is often linked to mutation. The test serves as a quick and convenient assay to estimate the carcinogenic potential of a compound because standard carcinogen assays on mice and rats are time-consuming and expensive. However, false-positives and false-negatives are known.

A carcinogen is any substance, radionuclide, or radiation that promotes carcinogenesis, the formation of cancer. This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive substances are considered carcinogens, but their carcinogenic activity is attributed to the radiation, for example gamma rays and alpha particles, which they emit. Common examples of non-radioactive carcinogens are inhaled asbestos, certain dioxins, and tobacco smoke. Although the public generally associates carcinogenicity with synthetic chemicals, it is equally likely to arise in both natural and synthetic substances. Carcinogens are not necessarily immediately toxic; thus, their effect can be insidious.

In genetics, a mutagen is a physical or chemical agent that permanently changes genetic material, usually DNA, in an organism and thus increases the frequency of mutations above the natural background level. As many mutations can cause cancer, such mutagens are therefore carcinogens, although not all necessarily are. All mutagens have characteristic mutational signatures with some chemicals becoming mutagenic through cellular processes. Not all mutations are caused by mutagens: so-called "spontaneous mutations" occur due to spontaneous hydrolysis, errors in DNA replication, repair and recombination.

Formaldehyde ( fer-mal-duh-hahyd, alsofor-) (systematic name methanal) is a naturally occurring organic compound with the formula CH2O (H−CHO). The pure compound is a pungent-smelling colorless gas that polymerises spontaneously into paraformaldehyde (refer to section Forms below), hence it is stored as an aqueous solution (formalin). It is the simplest of the aldehydes (R−CHO). The common name of this substance comes from its similarity and relation to formic acid.
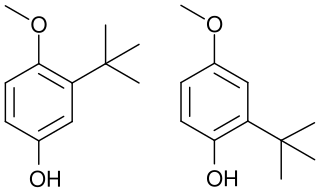
Butylated hydroxyanisole (BHA) is an antioxidant consisting of a mixture of two isomeric organic compounds, 2-tert-butyl-4-hydroxyanisole and 3-tert-butyl-4-hydroxyanisole. It is prepared from 4-methoxyphenol and isobutylene. It is a waxy solid used as a food additive with the E number E320. The primary use for BHA is as an antioxidant and preservative in food, food packaging, animal feed, cosmetics, rubber, and petroleum products. BHA also is commonly used in medicines, such as cholecalciferol, isotretinoin, lovastatin, and simvastatin, among others.

Acesulfame potassium, also known as acesulfame K or Ace K, is a synthetic calorie-free sugar substitute often marketed under the trade names Sunett and Sweet One. In the European Union, it is known under the E number E950. It was discovered accidentally in 1967 by German chemist Karl Clauss at Hoechst AG. In chemical structure, acesulfame potassium is the potassium salt of 6-methyl-1,2,3-oxathiazine-4(3H)-one 2,2-dioxide. It is a white crystalline powder with molecular formula C
4H
4KNO
4S and a molecular weight of 201.24 g/mol.

Ethyl carbamate (also called urethane) is an organic compound with the formula CH3CH2OC(O)NH2. It is an ester of carbamic acid and a white solid. Despite its name, it is not a component of polyurethanes. Because it is a carcinogen, it is little used, but it naturally forms in low quantities in many types of fermented foods and drinks.

Ethidium bromide is an intercalating agent commonly used as a fluorescent tag in molecular biology laboratories for techniques such as agarose gel electrophoresis. It is commonly abbreviated as EtBr, which is also an abbreviation for bromoethane. To avoid confusion, some laboratories have used the abbreviation EthBr for this salt. When exposed to ultraviolet light, it will fluoresce with an orange colour, intensifying almost 20-fold after binding to DNA. Under the name homidium, it has been commonly used since the 1950s in veterinary medicine to treat trypanosomiasis in cattle. The high incidence of antimicrobial resistance makes this treatment impractical in some areas, where the related isometamidium chloride is used instead. Although ethidium bromide is said to be mutagenic on some circumstances of exposure, evidence shows that it is relatively safe and its alleged high toxicity is largely a myth.
In genetics, genotoxicity describes the property of chemical agents that damages the genetic information within a cell causing mutations, which may lead to cancer. While genotoxicity is often confused with mutagenicity, all mutagens are genotoxic, whereas not all genotoxic substances are mutagenic. The alteration can have direct or indirect effects on the DNA: the induction of mutations, mistimed event activation, and direct DNA damage leading to mutations. The permanent, heritable changes can affect either somatic cells of the organism or germ cells to be passed on to future generations. Cells prevent expression of the genotoxic mutation by either DNA repair or apoptosis; however, the damage may not always be fixed leading to mutagenesis.

Phenylbutazone, often referred to as "bute", is a nonsteroidal anti-inflammatory drug (NSAID) for the short-term treatment of pain and fever in animals.

Bruce Nathan Ames is an American biochemist. He is a professor of Biochemistry and Molecular Biology Emeritus at the University of California, Berkeley, and was a senior scientist at Children's Hospital Oakland Research Institute (CHORI). He is the inventor of the Ames test, a system for easily and cheaply testing the mutagenicity of compounds.

Potassium bromate (KBrO3), is a bromate of potassium and takes the form of white crystals or powder. It is a strong oxidizing agent.

Ochratoxin A—a toxin produced by different Aspergillus and Penicillium species — is one of the most-abundant food-contaminating mycotoxins. It is also a frequent contaminant of water-damaged houses and of heating ducts. Human exposure can occur through consumption of contaminated food products, particularly contaminated grain and pork products, as well as coffee, wine grapes, and dried grapes. The toxin has been found in the tissues and organs of animals, including human blood and breast milk. Ochratoxin A, like most toxic substances, has large species- and sex-specific toxicological differences.
The artificial sweetener aspartame has been the subject of several controversies since its initial approval by the U.S. Food and Drug Administration (FDA) in 1974. The FDA approval of aspartame was highly contested, beginning with suspicions of its involvement in brain cancer, alleging that the quality of the initial research supporting its safety was inadequate and flawed, and that conflicts of interest marred the 1981 approval of aspartame, previously evaluated by two FDA panels that concluded to keep the approval on hold before further investigation. In 1987, the U.S. Government Accountability Office concluded that the food additive approval process had been followed properly for aspartame. The irregularities fueled a conspiracy theory, which the "Nancy Markle" email hoax circulated, along with claims—counter to the weight of medical evidence—that numerous health conditions are caused by the consumption of aspartame in normal doses.
Chemophobia is an aversion to or prejudice against chemicals or chemistry. The phenomenon has been ascribed both to a reasonable concern over the potential adverse effects of synthetic chemicals, and to an irrational fear of these substances because of misconceptions about their potential for harm, particularly the possibility of certain exposures to some synthetic chemicals elevating an individual's risk of cancer. Consumer products with labels such as "natural" and "chemical-free" appeal to chemophobic sentiments by offering consumers what appears to be a safer alternative.

Ractopamine is an animal feed additive used to promote leanness and increase food conversion efficiency in farmed animals in some countries, but banned in others. Pharmacologically, it is a phenol-based TAAR1 agonist and β adrenoreceptor agonist that stimulates β1 and β2 adrenergic receptors. It is most commonly administered to animals for meat production as ractopamine hydrochloride. It is the active ingredient in products marketed in the US as Paylean for swine, Optaflexx for cattle, and Topmax for turkeys. It was developed by Elanco Animal Health, a division of Eli Lilly and Company.

N-Nitrosodiethylamine (NDEA) is an organic compound with the formula Et2NNO (Et = C2H5). A member of the nitrosamines, it is a light-sensitive, volatile, clear yellow oil that is soluble in water, lipids, and other organic solvents. It has an amine or aromatic odor. It is used as gasoline and lubricant additive, antioxidant, and stabilizer for industry materials. When heated to decomposition, N-nitrosodiethylamine emits toxic fumes of nitrogen oxides. N-Nitrosodiethylamine affects DNA integrity, probably by alkylation, and is used in experimental research to induce liver tumorigenesis. It is carcinogenic and mutagenic. NDEA has also been found to perturb amino acid biosynthesis including arginine, as well as DNA damage repair and mitochondrial genome maintenance in yeast.

Dioxins and dioxin-like compounds (DLCs) are a group of chemical compounds that are persistent organic pollutants (POPs) in the environment. They are mostly by-products of burning or various industrial processes - or, in case of dioxin-like PCBs and PBBs, unwanted minor components of intentionally produced mixtures.

2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is a polychlorinated dibenzo-p-dioxin (sometimes shortened, though inaccurately, to simply 'dioxin') with the chemical formula C12H4Cl4O2. Pure TCDD is a colorless solid with no distinguishable odor at room temperature. It is usually formed as an unwanted product in burning processes of organic materials or as a side product in organic synthesis.

A bioassay is an analytical method to determine the concentration or potency of a substance by its effect on living animals or plants, or on living cells or tissues(in vitro). A bioassay can be either quantal or quantitative, direct or indirect. If the measured response is binary, the assay is quantal, if not, it is quantitative.

















