Experimental cancer treatments are mainstream medical therapies intended to treat cancer by improving on, supplementing or replacing conventional methods. However, researchers are still trying to determine whether these treatments are safe and effective treatments. Experimental cancer treatments are normally available only to people who participate in formal research programs, which are called clinical trials. Occasionally, a seriously ill person may be able to access an experimental drug through an expanded access program. Some of the treatments have regulatory approval for treating other conditions. Health insurance and publicly funded health care programs normally refuse to pay for experimental cancer treatments.

Telomerase, also called terminal transferase, is a ribonucleoprotein that adds a species-dependent telomere repeat sequence to the 3' end of telomeres. A telomere is a region of repetitive sequences at each end of the chromosomes of most eukaryotes. Telomeres protect the end of the chromosome from DNA damage or from fusion with neighbouring chromosomes. The fruit fly Drosophila melanogaster lacks telomerase, but instead uses retrotransposons to maintain telomeres.

The stromal cell-derived factor 1 (SDF-1), also known as C-X-C motif chemokine 12 (CXCL12), is a chemokine protein that in humans is encoded by the CXCL12 gene on chromosome 10. It is ubiquitously expressed in many tissues and cell types. Stromal cell-derived factors 1-alpha and 1-beta are small cytokines that belong to the chemokine family, members of which activate leukocytes and are often induced by proinflammatory stimuli such as lipopolysaccharide, TNF, or IL1. The chemokines are characterized by the presence of 4 conserved cysteines that form 2 disulfide bonds. They can be classified into 2 subfamilies. In the CC subfamily, the cysteine residues are adjacent to each other. In the CXC subfamily, they are separated by an intervening amino acid. The SDF1 proteins belong to the latter group. CXCL12 signaling has been observed in several cancers. The CXCL12 gene also contains one of 27 SNPs associated with increased risk of coronary artery disease.

Bufotalin is a cardiotoxic bufanolide steroid, cardiac glycoside analogue, secreted by a number of toad species. Bufotalin can be extracted from the skin parotoid glands of several types of toad.

Fumonisin B1 is the most prevalent member of a family of toxins, known as fumonisins, produced by several species of Fusarium molds, such as Fusarium verticillioides, which occur mainly in maize (corn), wheat and other cereals. Fumonisin B1 contamination of maize has been reported worldwide at mg/kg levels. Human exposure occurs at levels of micrograms to milligrams per day and is greatest in regions where maize products are the dietary staple.

The bafilomycins are a family of macrolide antibiotics produced from a variety of Streptomycetes. Their chemical structure is defined by a 16-membered lactone ring scaffold. Bafilomycins exhibit a wide range of biological activity, including anti-tumor, anti-parasitic, immunosuppressant and anti-fungal activity. The most used bafilomycin is bafilomycin A1, a potent inhibitor of cellular autophagy. Bafilomycins have also been found to act as ionophores, transporting potassium K+ across biological membranes and leading to mitochondrial damage and cell death.

Citrinin is a mycotoxin which is often found in food. It is a secondary metabolite produced by fungi that contaminates long-stored food and it causes different toxic effects, like nephrotoxic, hepatotoxic and cytotoxic effects. Citrinin is mainly found in stored grains, but sometimes also in fruits and other plant products.
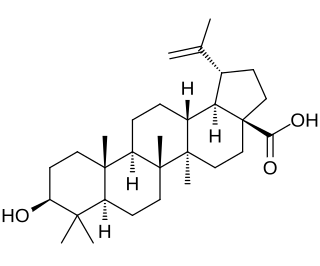
Betulinic acid is a naturally occurring pentacyclic triterpenoid which has antiretroviral, antimalarial, and anti-inflammatory properties, as well as a more recently discovered potential as an anticancer agent, by inhibition of topoisomerase. It is found in the bark of several species of plants, principally the white birch from which it gets its name, but also the ber tree, selfheal, the tropical carnivorous plants Triphyophyllum peltatum and Ancistrocladus heyneanus, Diospyros leucomelas, a member of the persimmon family, Tetracera boiviniana, the jambul, flowering quince, rosemary, and Pulsatilla chinensis.
Lycorine is a toxic crystalline alkaloid found in various Amaryllidaceae species, such as the cultivated bush lily, surprise lilies (Lycoris), and daffodils (Narcissus). It may be highly poisonous, or even lethal, when ingested in certain quantities. Regardless, it is sometimes used medicinally, a reason why some groups may harvest the very popular Clivia miniata.

Helenalin, or (-)-4-Hydroxy-4a,8-dimethyl-3,3a,4a,7a,8,9,9a-octahydroazuleno[6,5-b]furan-2,5-dione, is a toxic sesquiterpene lactone which can be found in several plants such as Arnica montana and Arnica chamissonis Helenalin is responsible for the toxicity of the Arnica spp. Although toxic, helenalin possesses some in vitro anti-inflammatory and anti-neoplastic effects. Helenalin can inhibit certain enzymes, such as 5-lipoxygenase and leukotriene C4 synthase. For this reason the compound or its derivatives may have potential medical applications.

Telomerase reverse transcriptase is a catalytic subunit of the enzyme telomerase, which, together with the telomerase RNA component (TERC), comprises the most important unit of the telomerase complex.
PIN2/TERF1-interacting telomerase inhibitor 1, also known as PINX1, is a human gene. PINX1 is also known as PIN2 interacting protein 1. PINX1 is a telomerase inhibitor and a possible tumor suppressor.

Arenobufagin is a cardiotoxic bufanolide steroid secreted by the Argentine toad Bufo arenarum. It has effects similar to digitalis, blocking the Na+/K+ pump in heart tissue.
Regorafenib, sold under the brand name Stivarga among others, is an oral multi-kinase inhibitor developed by Bayer which targets angiogenic, stromal and oncogenic receptor tyrosine kinase (RTK). Regorafenib shows anti-angiogenic activity due to its dual targeted VEGFR2-TIE2 tyrosine kinase inhibition. Since 2009 it was studied as a potential treatment option in multiple tumor types. By 2015 it had two US approvals for advanced cancers.

Stable nucleic acid lipid particles (SNALPs) are microscopic particles approximately 120 nanometers in diameter, smaller than the wavelengths of visible light. They have been used to deliver siRNAs therapeutically to mammals in vivo. In SNALPs, the siRNA is surrounded by a lipid bilayer containing a mixture of cationic and fusogenic lipids, coated with diffusible polyethylene glycol.

mTOR inhibitors are a class of drugs that inhibit the mammalian target of rapamycin (mTOR), which is a serine/threonine-specific protein kinase that belongs to the family of phosphatidylinositol-3 kinase (PI3K) related kinases (PIKKs). mTOR regulates cellular metabolism, growth, and proliferation by forming and signaling through two protein complexes, mTORC1 and mTORC2. The most established mTOR inhibitors are so-called rapalogs, which have shown tumor responses in clinical trials against various tumor types.

Dicycloplatin is a chemotherapy medication used to treat a number of cancers which includes the non-small-cell lung carcinoma and prostate cancer.

Isatuximab, sold under the brand name Sarclisa, is a monoclonal antibody (mAb) medication for the treatment of multiple myeloma.

4-Ipomeanol (4-IPO) is a pulmonary pre-toxin isolated from sweet potatoes infected with the fungus Fusarium solani. One of the 4-IPO metabolites is toxic to the lungs, liver and kidney in humans and animals. This metabolite can covalently bind to proteins, thereby interfering with normal cell processes.

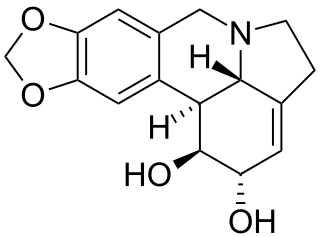
14-Hydroxygelsenicine (HGE) is a gelsedine-type indole alkaloid naturally found in some plants of the Gelsemium genus. G. elegans was used in traditional Chinese medicine as a remedy for a plethora of conditions such as skin ulcers and dermatitis, pain related to cancer, rheumatic arthritis, psoriasis as well as to treat bone fractures. It can also be found under the names “Duan Chang Cao”, “Gou Wen” and “heartbreak grass”. G. elegans is also known for its toxic effects; it is used by hilltribes of southeastern Asia as an effective means of committing suicide and has been linked to certain types of toxic honey, where HGE was the most abundant component. Gelsedine-type alkaloids from G. elegans usually express high toxicity, with gelsenicine being one of the most toxic. However, toxicity of HGE has not yet been thoroughly researched. More recent studies have shown that alkaloids derived from G. elegans have anti-tumor, anti-inflammatory, analgesic, and immunomodulation properties, with the toxic dose being close to the therapeutic dose.



















