
Garcinia is a genus of flowering plants in the family Clusiaceae native to Asia, America, Australia, tropical and southern Africa, and Polynesia. The number of species is disputed; the Kew Gardens recognise up to 400. Commonly, the plants in this genus are called saptrees, mangosteens, garcinias, or monkey fruit.
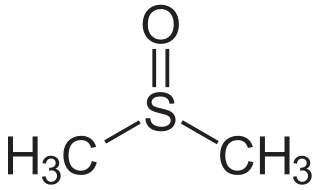
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula (CH3)2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water. It has a relatively high boiling point. DMSO has the unusual property that many individuals perceive a garlic-like taste in the mouth after DMSO makes contact with their skin.

Garcinia gummi-gutta is a tropical species of Garcinia native to South Asia and Southeast Asia. Common names include Garcinia cambogia, as well as brindle berry, and Malabar tamarind. The fruit looks like a small pumpkin and is green to pale yellow in color.

Gamboge is a partially transparent deep saffron to mustard yellow pigment. It is the traditional colour used to dye Buddhist monks' robes, and Theravada Buddhist monks in particular. Physicist Jean Perrin used this pigment to prove Brownian motion in 1908.

The Claisen rearrangement is a powerful carbon–carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen. The heating of an allyl vinyl ether will initiate a [3,3]-sigmatropic rearrangement to give a γ,δ-unsaturated carbonyl.

Xanthone is an organic compound with the molecular formula O[C6H4]2CO. It is a white solid.

Mangostin is a natural xanthonoid, a type of organic compound isolated from various parts of the mangosteen tree. It is a yellow crystalline solid with a xanthone core structure. Mangostin and a variety of other xanthonoids from mangosteen have been investigated for biological properties including antioxidant, anti-bacterial, anti-inflammatory, and anticancer activities.
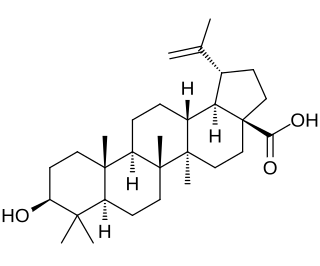
Betulinic acid is a naturally occurring pentacyclic triterpenoid which has antiretroviral, antimalarial, and anti-inflammatory properties, as well as a more recently discovered potential as an anticancer agent, by inhibition of topoisomerase. It is found in the bark of several species of plants, principally the white birch from which it gets its name, but also the ber tree, selfheal, the tropical carnivorous plants Triphyophyllum peltatum and Ancistrocladus heyneanus, Diospyros leucomelas, a member of the persimmon family, Tetracera boiviniana, the jambul, flowering quince, rosemary, and Pulsatilla chinensis.

Hydroxycitric acid (HCA) is a derivative of citric acid that is found in a variety of tropical plants including Garcinia cambogia and Hibiscus sabdariffa.
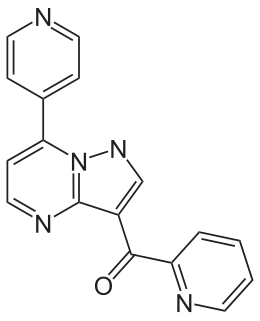
Ocinaplon is an anxiolytic drug in the pyrazolopyrimidine family of drugs. Other pyrazolopyrimidine drugs include zaleplon and indiplon.

Garcinia morella is a species of tree in the family Clusiaceae found in India, and Sri Lanka.

A xanthonoid is a chemical natural phenolic compound formed from the xanthone backbone. Many members of the Clusiaceae contain xanthonoids.

Gambogic acid is a xanthonoid that is derived from the brownish or orange resin from Garcinia hanburyi. Garcinia hanburyi is a small to medium-sized evergreen tree with smooth grey bark. It is native to Cambodia, southern Vietnam, and Thailand and has been successfully introduced in Singapore.
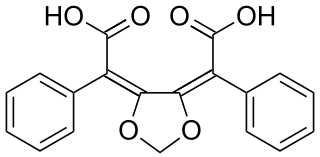
Ustalic acid is a naturally occurring chemical compound found in the poisonous mushroom Tricholoma ustale.

Hypericum ericoides is a dwarf shrub in the flowering plant family Hypericaceae, section Coridium. It is found in eastern and southeastern Spain, Morocco, and Tunisia. Its preferred habitat is fissures in calcareous rocks in warm, sunny places, from 200 to 2,000 meters above sea level (MASL).

Acetalated dextran is a biodegradable polymer based on dextran that has acetal modified hydroxyl groups. After synthesis, the hydrophilic polysaccharide dextran is rendered insoluble in water, but soluble in organic solvents. This allows it to be processed in the same manner as many polyesters, like poly(lactic-co-glycolic acid), through processes like solvent evaporation and emulsion. Acetalated dextran is structurally different from acetylated dextran.

The phomoxanthones are a loosely defined class of natural products. The two founding members of this class are phomoxanthone A and phomoxanthone B. Other compounds were later also classified as phomoxanthones, although a unifying nomenclature has not yet been established. The structure of all phomoxanthones is derived from a dimer of two covalently linked tetrahydroxanthones, and they differ mainly in the position of this link as well as in the acetylation status of their hydroxy groups. The phomoxanthones are structurally closely related to other tetrahydroxanthone dimers such as the secalonic acids and the eumitrins. While most phomoxanthones were discovered in fungi of the genus Phomopsis, most notably in the species Phomopsis longicolla, some have also been found in Penicillium sp.
Garcinia forbesii, commonly known as the rose kandis or just kandis, is a small to medium-sized tree in the family Clusiaceae or Guttiferae. The specific epithet (forbesii) honors Scottish naturalist Henry Ogg Forbes.

Garcinia pseudoguttifera, known as the mo'onia tree in its native range, is a species of flowering tree in the family Clusiaceae or Guttiferae.

Lichexanthone is an organic compound in the structural class of chemicals known as xanthones. Lichexanthone was first isolated and identified by Japanese chemists from a species of leafy lichen in the 1940s. The compound is known to occur in many lichens, and it is important in the taxonomy of species in several genera, such as Pertusaria and Pyxine. More than a dozen lichen species have a variation of the word lichexanthone incorporated as part of their binomial name. The presence of lichexanthone in lichens causes them to fluoresce a greenish-yellow colour under long-wavelength UV light; this feature is used to help identify some species. Lichexanthone is also found in several plants, and some species of fungi that do not form lichens.



















