
Lipids are a broad group of organic compounds which include fats, waxes, sterols, fat-soluble vitamins, monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include storing energy, signaling, and acting as structural components of cell membranes. Lipids have applications in the cosmetic and food industries, and in nanotechnology.

Nicotinamide adenine dinucleotide phosphate, abbreviated NADP or, in older notation, TPN (triphosphopyridine nucleotide), is a cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require NADPH as a reducing agent ('hydrogen source'). NADPH is the reduced form, whereas NADP+ is the oxidized form. NADP+ is used by all forms of cellular life. NADP+ is essential for life because it is needed for cellular respiration.
Biosynthesis, i.e., chemical synthesis occurring in biological contexts, is a term most often referring to multi-step, enzyme-catalyzed processes where chemical substances absorbed as nutrients serve as enzyme substrates, with conversion by the living organism either into simpler or more complex products. Examples of biosynthetic pathways include those for the production of amino acids, lipid membrane components, and nucleotides, but also for the production of all classes of biological macromolecules, and of acetyl-coenzyme A, adenosine triphosphate, nicotinamide adenine dinucleotide and other key intermediate and transactional molecules needed for metabolism. Thus, in biosynthesis, any of an array of compounds, from simple to complex, are converted into other compounds, and so it includes both the catabolism and anabolism of complex molecules. Biosynthetic processes are often represented via charts of metabolic pathways. A particular biosynthetic pathway may be located within a single cellular organelle, while others involve enzymes that are located across an array of cellular organelles and structures.
Fatty acid metabolism consists of various metabolic processes involving or closely related to fatty acids, a family of molecules classified within the lipid macronutrient category. These processes can mainly be divided into (1) catabolic processes that generate energy and (2) anabolic processes where they serve as building blocks for other compounds.
Dihydroxyacetone phosphate (DHAP, also glycerone phosphate in older texts) is the anion with the formula HOCH2C(O)CH2OPO32-. This anion is involved in many metabolic pathways, including the Calvin cycle in plants and glycolysis. It is the phosphate ester of dihydroxyacetone.
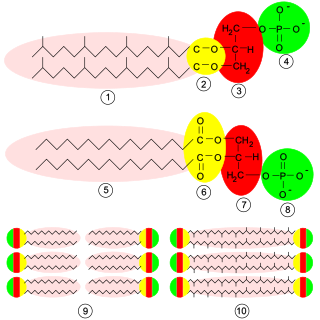
Glycerophospholipids or phosphoglycerides are glycerol-based phospholipids. They are the main component of biological membranes in eukaryotic cells. They are a type of lipid, of which its composition affects membrane structure and properties. Two major classes are known: those for bacteria and eukaryotes and a separate family for archaea.

Plasmalogens are a class of glycerophospholipid with a plasmenyl group linked to a lipid at the sn-2 position of the glycerol backbone. Plasmalogens are found in multiple domains of life, including mammals, invertebrates, protozoa, and anaerobic bacteria. They are commonly found in cell membranes in the nervous, immune, and cardiovascular systems. In humans, lower levels of plasmalogens are studied in relation to some diseases. Plasmalogens are also associated with adaptations to extreme environments in non-human organisms.

In biochemistry, an ether lipid refers to any lipid in which the lipid "tail" group is attached to the glycerol backbone via an ether bond at any position. In contrast, conventional glycerophospholipids and triglycerides are triesters. Structural types include:
Lipid metabolism is the synthesis and degradation of lipids in cells, involving the breakdown and storage of fats for energy and the synthesis of structural and functional lipids, such as those involved in the construction of cell membranes. In animals, these fats are obtained from food and are synthesized by the liver. Lipogenesis is the process of synthesizing these fats. The majority of lipids found in the human body from ingesting food are triglycerides and cholesterol. Other types of lipids found in the body are fatty acids and membrane lipids. Lipid metabolism is often considered the digestion and absorption process of dietary fat; however, there are two sources of fats that organisms can use to obtain energy: from consumed dietary fats and from stored fat. Vertebrates use both sources of fat to produce energy for organs such as the heart to function. Since lipids are hydrophobic molecules, they need to be solubilized before their metabolism can begin. Lipid metabolism often begins with hydrolysis, which occurs with the help of various enzymes in the digestive system. Lipid metabolism also occurs in plants, though the processes differ in some ways when compared to animals. The second step after the hydrolysis is the absorption of the fatty acids into the epithelial cells of the intestinal wall. In the epithelial cells, fatty acids are packaged and transported to the rest of the body.
sn-Glycerol 3-phosphate is the organic ion with the formula HOCH2CH(OH)CH2OPO32-. It is one of two stereoisomers of the ester of dibasic phosphoric acid (HOPO32-) and glycerol. It is a component of bacterial and eukaryotic glycerophospholipids. From a historical reason, it is also known as L-glycerol 3-phosphate, D-glycerol 1-phosphate, L-α-glycerophosphoric acid.

The glycerol-3-phosphate shuttle is a mechanism used in skeletal muscle and the brain that regenerates NAD+ from NADH, a by-product of glycolysis. NADH is a reducing equivalent that stores electrons generated in the cytoplasm during glycolysis. NADH must be transported into the mitochondria to enter the oxidative phosphorylation pathway. However, the inner mitochondrial membrane is impermeable to NADH and only contains a transport system for NAD+. Depending on the type of tissue either the glycerol-3-phosphate shuttle pathway or the malate–aspartate shuttle pathway is used to transport electrons from cytoplasmic NADH into the mitochondria.

Glycerol-3-phosphate dehydrogenase (GPDH) is an enzyme that catalyzes the reversible redox conversion of dihydroxyacetone phosphate to sn-glycerol 3-phosphate.

Phosphatidylglycerol is a glycerophospholipid found in pulmonary surfactant and in the plasma membrane where it directly activates lipid-gated ion channels.
In enzymology, a sn-glycerol-1-phosphate dehydrogenase (EC 1.1.1.261) is an enzyme that catalyzes the chemical reaction
In enzymology, a 1,3-propanediol dehydrogenase (EC 1.1.1.202) is an enzyme that catalyzes the chemical reaction
In enzymology, a glycerol-3-phosphate dehydrogenase [NAD(P)+] (EC 1.1.1.94) is an enzyme that catalyzes the chemical reaction
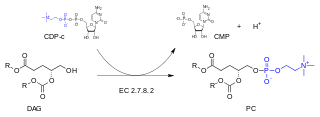
In enzymology, a diacylglycerol cholinephosphotransferase is an enzyme that catalyzes the chemical reaction
Archaeol is a diether composed of two phytanyl chains linked to the sn-2 and sn-3 positions of glycerol. As its phosphate ester, it is a common component of the membranes of archaea.
Glycerol-3-phosphate dehydrogenase (EC 1.1.5.3 is an enzyme with systematic name sn-glycerol 3-phosphate:quinone oxidoreductase. This enzyme catalyses the following chemical reaction
CDP-archaeol synthase is an enzyme with systematic name CTP:2,3-bis-O-(geranylgeranyl)-sn-glycero-1-phosphate cytidylyltransferase. This enzyme catalyses the following chemical reaction










