
The haloalkanes are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes that contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula "RX" where R is an alkyl or substituted alkyl group and X is a halogen.
In chemistry, halogenation is a chemical reaction which introduces of one or more halogens into a chemical compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens. Halides are also commonly introduced using salts of the halides and halogen acids. Many specialized reagents exist for and introducing halogens into diverse substrates, e.g. thionyl chloride.
A halohydrin dehalogenase is an enzyme involved in the bacterial degradation of vicinal halohydrins. In several species of bacteria, it catalyses the dehalogenation of halohydrins to produce the corresponding epoxides. Different isoforms of the enzyme fall into one of three groups, A, B or C. Halogenases of the same class are genetically similar, but differ greatly from halogenases from a different group. Currently the most well-studied isoform is HheC which is purified from the bacterial species Agrobacterium radiobacter. The ability to dehalogenate organic compounds as well as form enantiomeric selective epoxides have generated interest in the potential of this enzyme in the biochemical field.
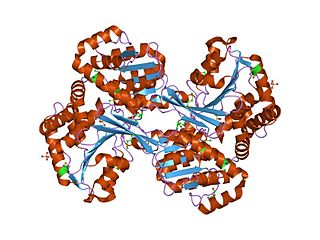
In enzymology, histidinol dehydrogenase (HIS4) (HDH) (EC 1.1.1.23) is an enzyme that catalyzes the chemical reaction
In enzymology, an alkane 1-monooxygenase (EC 1.14.15.3) is an enzyme that catalyzes the chemical reactions
Acireductone dioxygenase (Ni2+-requiring) (EC 1.13.11.53) is an enzyme that catalyzes the chemical reaction
In enzymology, a lignin peroxidase (EC 1.11.1.14) is an enzyme that catalyzes the chemical reaction

In enzymology, a L-ribulose-5-phosphate 4-epimerase is an enzyme that catalyzes the interconversion of ribulose 5-phosphate and xylulose 5-phosphate in the oxidative phase of the Pentose phosphate pathway.
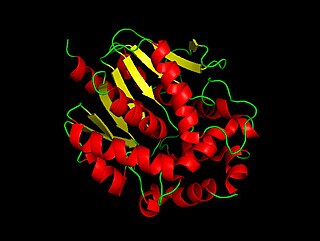
In enzymology, a haloalkane dehalogenase (EC 3.8.1.5) is an enzyme that catalyzes the chemical reaction
In enzymology, a (R)-2-haloacid dehalogenase(EC 3.8.1.9), DL-2-haloacid halidohydrolase (inversion of configuration), DL-DEXi, (R,S)-2-haloacid dehalogenase (configuration-inverting)) is an enzyme that catalyzes the chemical reaction
In enzymology, a (S)-2-haloacid dehalogenase (EC 3.8.1.2) is an enzyme that catalyzes the chemical reaction
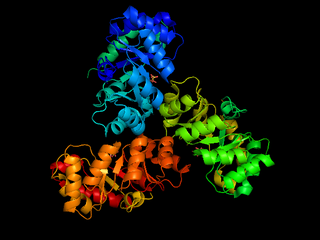
The enzyme 2-dehydro-3-deoxy-phosphogluconate aldolase, commonly known as KDPG aldolase, catalyzes the chemical reaction
In enzymology, a choloylglycine hydrolase (EC 3.5.1.24) is an enzyme that catalyzes the chemical reaction

In enzymology, a polynucleotide adenylyltransferase is an enzyme that catalyzes the chemical reaction
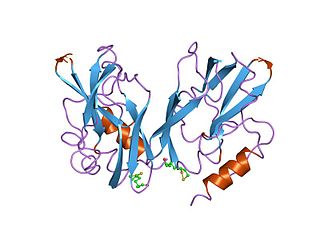
The glycine cleavage system (GCS) is also known as the glycine decarboxylase complex or GDC. The system is a series of enzymes that are triggered in response to high concentrations of the amino acid glycine. The same set of enzymes is sometimes referred to as glycine synthase when it runs in the reverse direction to form glycine. The glycine cleavage system is composed of four proteins: the T-protein, P-protein, L-protein, and H-protein. They do not form a stable complex, so it is more appropriate to call it a "system" instead of a "complex". The H-protein is responsible for interacting with the three other proteins and acts as a shuttle for some of the intermediate products in glycine decarboxylation. In both animals and plants, the glycine cleavage system is loosely attached to the inner membrane of the mitochondria. Mutations in this enzymatic system are linked with glycine encephalopathy.
In organic chemistry, dehalogenation is a set of chemical reactions that involve the cleavage of carbon-halogen bonds; as such, it is the inverse reaction of halogenation. Dehalogenations come in many varieties, including defluorination, dechlorination, debromination, and deiodination. Incentives to investigate dehalogenations include both constructive and destructive goals. Complicated organic compounds such as pharmaceutical drugs are occasionally generated by dehalogenation. Many organohalides are hazardous, so their dehalogenation is one route for their detoxification.

2,6-Dihydroxypyridine is an alkaloid with the molecular formula C5H3N(OH)2. It is a colorless solid. 2,6-Dihyroxypyridine is an intermediate in the degradation of nicotine.
A dehalogenase is a type of enzyme that catalyzes the removal of a halogen atom from a substrate.
In chemistry, molecular oxohalides (oxyhalides) are a group of chemical compounds in which both oxygen and halogen atoms are attached to another chemical element A in a single molecule. They have the general formula AOmXn, where X is a halogen. Known oxohalides have fluorine (F), chlorine (Cl), bromine (Br), and/or iodine (I) in their molecules. The element A may be a main group element, a transition element, a rare earth element or an actinide. The term oxohalide, or oxyhalide, may also refer to minerals and other crystalline substances with the same overall chemical formula, but having an ionic structure.
Adsorbable organic halides (AOX) is a measure of the organic halogen load at a sampling site such as soil from a land fill, water, or sewage waste. The procedure measures chlorine, bromine, and iodine as equivalent halogens, but does not measure fluorine levels in the sample.









