
The enzyme-linked immunosorbent assay (ELISA) is a commonly used analytical biochemistry assay, first described by Eva Engvall and Peter Perlmann in 1971. The assay is a solid-phase type of enzyme immunoassay (EIA) to detect the presence of a ligand in a liquid sample using antibodies directed against the ligand to be measured. ELISA has been used as a diagnostic tool in medicine, plant pathology, and biotechnology, as well as a quality control check in various industries.
An assay is an investigative (analytic) procedure in laboratory medicine, mining, pharmacology, environmental biology and molecular biology for qualitatively assessing or quantitatively measuring the presence, amount, or functional activity of a target entity. The measured entity is often called the analyte, the measurand, or the target of the assay. The analyte can be a drug, biochemical substance, chemical element or compound, or cell in an organism or organic sample. An assay usually aims to measure an analyte's intensive property and express it in the relevant measurement unit.

A radioimmunoassay (RIA) is an immunoassay that uses radiolabeled molecules in a stepwise formation of immune complexes. A RIA is a very sensitive in vitro assay technique used to measure concentrations of substances, usually measuring antigen concentrations by use of antibodies.
An immunoassay (IA) is a biochemical test that measures the presence or concentration of a macromolecule or a small molecule in a solution through the use of an antibody (usually) or an antigen (sometimes). The molecule detected by the immunoassay is often referred to as an "analyte" and is in many cases a protein, although it may be other kinds of molecules, of different sizes and types, as long as the proper antibodies that have the required properties for the assay are developed. Analytes in biological liquids such as serum or urine are frequently measured using immunoassays for medical and research purposes.
A protein microarray is a high-throughput method used to track the interactions and activities of proteins, and to determine their function, and determining function on a large scale. Its main advantage lies in the fact that large numbers of proteins can be tracked in parallel. The chip consists of a support surface such as a glass slide, nitrocellulose membrane, bead, or microtitre plate, to which an array of capture proteins is bound. Probe molecules, typically labeled with a fluorescent dye, are added to the array. Any reaction between the probe and the immobilised protein emits a fluorescent signal that is read by a laser scanner. Protein microarrays are rapid, automated, economical, and highly sensitive, consuming small quantities of samples and reagents. The concept and methodology of protein microarrays was first introduced and illustrated in antibody microarrays in 1983 in a scientific publication and a series of patents. The high-throughput technology behind the protein microarray was relatively easy to develop since it is based on the technology developed for DNA microarrays, which have become the most widely used microarrays.
Immunoprecipitation (IP) is the technique of precipitating a protein antigen out of solution using an antibody that specifically binds to that particular protein. This process can be used to isolate and concentrate a particular protein from a sample containing many thousands of different proteins. Immunoprecipitation requires that the antibody be coupled to a solid substrate at some point in the procedure.
Protein methods are the techniques used to study proteins. There are experimental methods for studying proteins. Computational methods typically use computer programs to analyze proteins. However, many experimental methods require computational analysis of the raw data.
Heterophile antibodies are antibodies induced by external antigens.

Antibody-dependent cellular cytotoxicity (ADCC), also referred to as antibody-dependent cell-mediated cytotoxicity, is a mechanism of cell-mediated immune defense whereby an effector cell of the immune system kills a target cell, whose membrane-surface antigens have been bound by specific antibodies. It is one of the mechanisms through which antibodies, as part of the humoral immune response, can act to limit and contain infection.

Radioactivity is generally used in life sciences for highly sensitive and direct measurements of biological phenomena, and for visualizing the location of biomolecules radiolabelled with a radioisotope.

A lateral flow test (LFT), is an assay also known as a lateral flow device (LFD), lateral flow immunochromatographic assay, or rapid test. It is a simple device intended to detect the presence of a target substance in a liquid sample without the need for specialized and costly equipment. LFTs are widely used in medical diagnostics in the home, at the point of care, and in the laboratory. For instance, the home pregnancy test is an LFT that detects a specific hormone. These tests are simple and economical and generally show results in around five to thirty minutes. Many lab-based applications increase the sensitivity of simple LFTs by employing additional dedicated equipment. Because the target substance is often a biological antigen, many lateral flow tests are rapid antigen tests.
Proliferation, as one of the hallmarks and most fundamental biological processes in tumors, is associated with tumor progression, response to therapy, and cancer patient survival. Consequently, the evaluation of a tumor proliferative index has clinical significance in characterizing many solid tumors and hematologic malignancies. This has led investigators to develop different technologies to evaluate the proliferation index in tumor samples. The most commonly used methods in evaluating a proliferative index include mitotic indexing, thymidine-labeling index, bromodeoxyuridine assay, the determination of fraction of cells in various phases of cell cycle, and the immunohistochemical evaluation of cell cycle-associated proteins.
Magnetic immunoassay (MIA) is a type of diagnostic immunoassay using magnetic beads as labels in lieu of conventional enzymes (ELISA), radioisotopes (RIA) or fluorescent moieties to detect a specified analyte. MIA involves the specific binding of an antibody to its antigen, where a magnetic label is conjugated to one element of the pair. The presence of magnetic beads is then detected by a magnetic reader (magnetometer) which measures the magnetic field change induced by the beads. The signal measured by the magnetometer is proportional to the analyte concentration in the initial sample.
Indium-111 (111In) is a radioactive isotope of indium (In). It decays by electron capture to stable cadmium-111 with a half-life of 2.8 days. Indium-111 chloride (111InCl) solution is produced by proton irradiation of a cadmium target in a cyclotron, as recommended by International Atomic Energy Agency (IAEA). The former method is more commonly used as it results in a high level of radionuclide purity.
Virus quantification is counting or calculating the number of virus particles (virions) in a sample to determine the virus concentration. It is used in both research and development (R&D) in academic and commercial laboratories as well as in production situations where the quantity of virus at various steps is an important variable that must be monitored. For example, the production of virus-based vaccines, recombinant proteins using viral vectors, and viral antigens all require virus quantification to continually monitor and/or modify the process in order to optimize product quality and production yields and to respond to ever changing demands and applications. Other examples of specific instances where viruses need to be quantified include clone screening, multiplicity of infection (MOI) optimization, and adaptation of methods to cell culture.
Mass spectrometric immunoassay (MSIA) is a rapid method is used to detect and/ or quantify antigens and or antibody analytes. This method uses an analyte affinity isolation to extract targeted molecules and internal standards from biological fluid in preparation for matrix assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF-MS). This method allows for "top down" and "bottom up" analysis. This sensitive method allows for a new and improved process for detecting multiple antigens and antibodies in a single assay. This assay is also capable of distinguishing mass shifted forms of the same molecule via a panantibody, as well as distinguish point mutations in proteins. Each specific form is detected uniquely based on their characteristic molecular mass. MSIA has dual specificity because of the antibody-antigen reaction coupled with the power of a mass spectrometer.
A ligand binding assay (LBA) is an assay, or an analytic procedure, which relies on the binding of ligand molecules to receptors, antibodies or other macromolecules. A detection method is used to determine the presence and extent of the ligand-receptor complexes formed, and this is usually determined electrochemically or through a fluorescence detection method. This type of analytic test can be used to test for the presence of target molecules in a sample that are known to bind to the receptor.

Fluorescence polarization immunoassay (FPIA) is a class of in vitro biochemical test used for rapid detection of antibody or antigen in sample. FPIA is a competitive homogenous assay, that consists of a simple prepare and read method, without the requirement of separation or washing steps.
The enzyme-linked immunosorbent spot (ELISpot) is a type of assay that focuses on quantitatively measuring the frequency of cytokine secretion for a single cell. The ELISpot Assay is also a form of immunostaining since it is classified as a technique that uses antibodies to detect a protein analyte, with the word analyte referring to any biological or chemical substance being identified or measured.
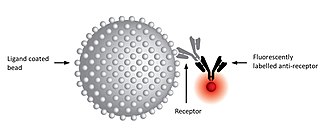
A kinetic exclusion assay (KinExA) is a type of bioassay in which a solution containing receptor, ligand, and receptor-ligand complex is briefly exposed to additional ligand immobilized on a solid phase.








