Related Research Articles

The enzyme-linked immunosorbent assay (ELISA) is a commonly used analytical biochemistry assay, first described by Eva Engvall and Peter Perlmann in 1971. The assay uses a solid-phase type of enzyme immunoassay (EIA) to detect the presence of a ligand in a liquid sample using antibodies directed against the protein to be measured. ELISA has been used as a diagnostic tool in medicine, plant pathology, and biotechnology, as well as a quality control check in various industries.

The western blot, or western blotting, is a widely used analytical technique in molecular biology and immunogenetics to detect specific proteins in a sample of tissue homogenate or extract.

Antinuclear antibodies are autoantibodies that bind to contents of the cell nucleus. In normal individuals, the immune system produces antibodies to foreign proteins (antigens) but not to human proteins (autoantigens). In some cases, antibodies to human antigens are produced.
Humoral immunity is the aspect of immunity that is mediated by macromolecules - including secreted antibodies, complement proteins, and certain antimicrobial peptides - located in extracellular fluids. Humoral immunity is named so because it involves substances found in the humors, or body fluids. It contrasts with cell-mediated immunity. Humoral immunity is also referred to as antibody-mediated immunity.

In biochemistry, immunostaining is any use of an antibody-based method to detect a specific protein in a sample. The term "immunostaining" was originally used to refer to the immunohistochemical staining of tissue sections, as first described by Albert Coons in 1941. However, immunostaining now encompasses a broad range of techniques used in histology, cell biology, and molecular biology that use antibody-based staining methods.
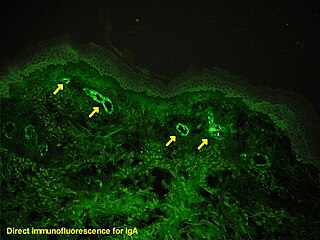
Immunofluorescence is a technique used for light microscopy with a fluorescence microscope and is used primarily on microbiological samples. This technique uses the specificity of antibodies to their antigen to target fluorescent dyes to specific biomolecule targets within a cell, and therefore allows visualization of the distribution of the target molecule through the sample. The specific region an antibody recognizes on an antigen is called an epitope. There have been efforts in epitope mapping since many antibodies can bind the same epitope and levels of binding between antibodies that recognize the same epitope can vary. Additionally, the binding of the fluorophore to the antibody itself cannot interfere with the immunological specificity of the antibody or the binding capacity of its antigen. Immunofluorescence is a widely used example of immunostaining and is a specific example of immunohistochemistry. This technique primarily makes use of fluorophores to visualise the location of the antibodies.
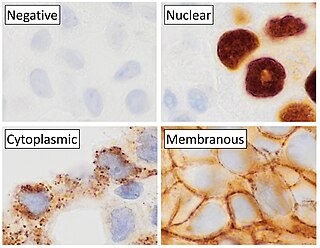
Immunohistochemistry (IHC) is the most common application of immunostaining. It involves the process of selectively identifying antigens (proteins) in cells of a tissue section by exploiting the principle of antibodies binding specifically to antigens in biological tissues. IHC takes its name from the roots "immuno", in reference to antibodies used in the procedure, and "histo", meaning tissue. Albert Coons conceptualized and first implemented the procedure in 1941.
Serology is the scientific study of serum and other body fluids. In practice, the term usually refers to the diagnostic identification of antibodies in the serum. Such antibodies are typically formed in response to an infection, against other foreign proteins, or to one's own proteins. In either case, the procedure is simple.
Titer or titre is a way of expressing concentration. Titer testing employs serial dilution to obtain approximate quantitative information from an analytical procedure that inherently only evaluates as positive or negative. The titre corresponds to the highest dilution factor that still yields a positive reading. For example, positive readings in the first 8 serial, twofold dilutions translate into a titer of 1:256. Titres are sometimes expressed by the denominator only, for example 1:256 is written 256.
A Coombs test, also known as antiglobulin test (AGT), is either of two blood tests used in immunohematology. They are the direct and indirect Coombs tests. The direct Coombs test detects antibodies that are stuck to the surface of the red blood cells. Since these antibodies sometimes destroy red blood cells, a person can be anemic and this test can help clarify the condition. The indirect Coombs detects antibodies that are floating freely in the blood. These antibodies could act against certain red blood cells and the test can be done to diagnose reactions to a blood transfusion.
Hemagglutination, or haemagglutination, is a specific form of agglutination that involves red blood cells (RBCs). It has two common uses in the laboratory: blood typing and the quantification of virus dilutions in a haemagglutination assay.
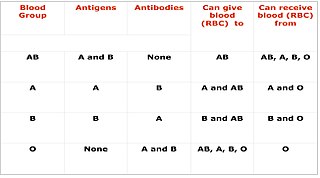
Cross-matching or crossmatching is a test performed before a blood transfusion as part of blood compatibility testing. Normally, this involves adding the recipient's blood plasma to a sample of the donor's red blood cells. If the blood is incompatible, the antibodies in the recipient's plasma will bind to antigens on the donor red blood cells. This antibody-antigen reaction can be detected through visible clumping or destruction of the red blood cells, or by reaction with anti-human globulin. Along with blood typing of the donor and recipient and screening for unexpected blood group antibodies, cross-matching is one of a series of steps in pre-transfusion testing. In some circumstances, an electronic cross-match can be performed by comparing records of the recipient's ABO and Rh blood type against that of the donor sample. In emergencies, blood may be issued before cross-matching is complete. Cross-matching is also used to determine compatibility between a donor and recipient in organ transplantation.
The Weil–Felix test is an agglutination test for the diagnosis of rickettsial infections. It was first described in 1916. By virtue of its long history and of its simplicity, it has been one of the most widely employed tests for rickettsia on a global scale, despite being superseded in many settings by more sensitive and specific diagnostic tests. The Weil-Felix antibody was recently found to target rickettsia LPS O-antigen.
Autoimmune hemolytic anemia (AIHA) occurs when antibodies directed against the person's own red blood cells (RBCs) cause them to burst (lyse), leading to an insufficient number of oxygen-carrying red blood cells in the circulation. The lifetime of the RBCs is reduced from the normal 100–120 days to just a few days in serious cases. The intracellular components of the RBCs are released into the circulating blood and into tissues, leading to some of the characteristic symptoms of this condition. The antibodies are usually directed against high-incidence antigens, therefore they also commonly act on allogenic RBCs. AIHA is a relatively rare condition, with an incidence of 5-10 cases per 1 million persons per year in the warm-antibody type and 0.45 to 1.9 cases per 1 million persons per year in the cold antibody type. Autoimmune hemolysis might be a precursor of later onset systemic lupus erythematosus.
Extractable nuclear antigens (ENAs) are over 100 different soluble cytoplasmic and nuclear antigens. They are known as "extractable" because they can be removed from cell nuclei using saline and represent six main proteins: Ro, La, Sm, RNP, Scl-70, Jo1. Most ENAs are part of spliceosomes or nucleosomes complexes and are a type of small nuclear ribonucleoprotein (snRNPS). The location in the nucleus and association with spliceosomes or nucleosomes results in these ENAs being associated with additional RNA and proteins such as polymerases. This quality of ENAs often makes it difficult to purify and quantify their presence for clinical use.
A latex fixation test, also called a latex agglutination assay or test, is an assay used clinically in the identification and typing of many important microorganisms. These tests use the patient's antigen-antibody immune response. This response occurs when the body detects a pathogen and forms an antibody specific to an identified antigen present on the surface of the pathogen.
A Sabin–Feldman dye test is a serologic test to diagnose for toxoplasmosis. Patient serum is treated with Toxoplasma trophozoites and complement, and then incubated. After incubation, methylene blue is added. If anti-Toxo antibodies are present in the serum, the antibody-antigen complex activates complement to lyse the parasite membrane, Toxoplasma trophozoites are not stained ; if there are no antibodies, trophozoites with intact membrane are stained and appear blue under microscope . The dilution of the test serum at which 50% of the tachyzoites are thin, distorted and colorless is reported as antibody titer of the test serum. The test is highly sensitive and specific with no false positives reported so far.

Anti-double stranded DNA (Anti-dsDNA) antibodies are a group of anti-nuclear antibodies (ANA) the target antigen of which is double stranded DNA. Blood tests such as enzyme-linked immunosorbent assay (ELISA) and immunofluorescence are routinely performed to detect anti-dsDNA antibodies in diagnostic laboratories. They are highly diagnostic of systemic lupus erythematosus (SLE) and are implicated in the pathogenesis of lupus nephritis.
Virus quantification involves counting the number of viruses in a specific volume to determine the virus concentration. It is used in both research and development (R&D) in commercial and academic laboratories as well as production situations where the quantity of virus at various steps is an important variable. For example, the production of viral vaccines, recombinant proteins using viral vectors and viral antigens all require virus quantification to continually adapt and monitor the process in order to optimize production yields and respond to ever changing demands and applications. Examples of specific instances where known viruses need to be quantified include clone screening, multiplicity of infection (MOI) optimization and adaptation of methods to cell culture. This page discusses various techniques currently used to quantify viruses in liquid samples. These methods are separated into two categories, traditional vs. modern methods. Traditional methods are industry-standard methods that have been used for decades but are generally slow and labor-intensive. Modern methods are relatively new commercially available products and kits that greatly reduce quantification time. This is not meant to be an exhaustive review of all potential methods, but rather a representative cross-section of traditional methods and new, commercially available methods. While other published methods may exist for virus quantification, non-commercial methods are not discussed here.

Blood compatibility testing is conducted in a medical laboratory to identify potential incompatibilities between blood group systems in blood transfusion. It is also used to diagnose and prevent some complications of pregnancy that can occur when the baby has a different blood group from the mother. Blood compatibility testing includes blood typing, which detects the antigens on red blood cells that determine a person's blood type; testing for unexpected antibodies against blood group antigens ; and, in the case of blood transfusions, mixing the recipient's plasma with the donor's red blood cells to detect incompatibilities (crossmatching). Routine blood typing involves determining the ABO and RhD type, and involves both identification of ABO antigens on red blood cells and identification of ABO antibodies in the plasma. Other blood group antigens may be tested for in specific clinical situations.
References
- 1 2 "Animation Quiz 4 - Complement Fixation Test". highered.mheducation.com.
- ↑ C. Vaman Rao (2005). Immunology: a textbook. Alpha Science Int'l Ltd. pp. 112–. ISBN 978-1-84265-255-8 . Retrieved 3 December 2010.