
An antibody (Ab) is the secreted form of a B cell receptor; the term immunoglobulin (Ig) can refer to either the membrane-bound form or the secreted form of the B cell receptor, but they are, broadly speaking, the same protein, and so the terms are often treated as synonymous. Antibodies are large, Y-shaped proteins belonging to the immunoglobulin superfamily which are used by the immune system to identify and neutralize foreign objects such as bacteria and viruses, including those that cause disease. Antibodies can recognize virtually any size antigen with diverse chemical compositions from molecules. Each antibody recognizes one or more specific antigens. This term literally means "antibody generator", as it is the presence of an antigen that drives the formation of an antigen-specific antibody. Each tip of the "Y" of an antibody contains a paratope that specifically binds to one particular epitope on an antigen, allowing the two molecules to bind together with precision. Using this mechanism, antibodies can effectively "tag" a microbe or an infected cell for attack by other parts of the immune system, or can neutralize it directly.

The enzyme-linked immunosorbent assay (ELISA) is a commonly used analytical biochemistry assay, first described by Eva Engvall and Peter Perlmann in 1971. The assay is a solid-phase type of enzyme immunoassay (EIA) to detect the presence of a ligand in a liquid sample using antibodies directed against the protein to be measured. ELISA has been used as a diagnostic tool in medicine, plant pathology, and biotechnology, as well as a quality control check in various industries.
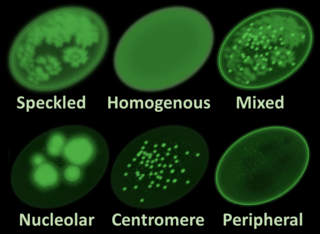
Antinuclear antibodies are autoantibodies that bind to contents of the cell nucleus. In normal individuals, the immune system produces antibodies to foreign proteins (antigens) but not to human proteins (autoantigens). In some cases, antibodies to human antigens are produced.
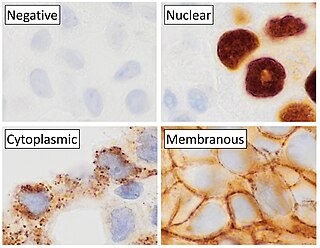
Immunohistochemistry (IHC) is a form of immunostaining. It involves the process of selectively identifying antigens (proteins) in cells and tissue, by exploiting the principle of antibodies binding specifically to antigens in biological tissues. Albert Hewett Coons, Ernest Berliner, Norman Jones and Hugh J Creech was the first to develop immunofluorescence in 1941. This led to the later development of immunohistochemistry.
Serology is the scientific study of serum and other body fluids. In practice, the term usually refers to the diagnostic identification of antibodies in the serum. Such antibodies are typically formed in response to an infection, against other foreign proteins, or to one's own proteins. In either case, the procedure is simple.
Polyclonal antibodies (pAbs) are antibodies that are secreted by different B cell lineages within the body. They are a collection of immunoglobulin molecules that react against a specific antigen, each identifying a different epitope.

Carcinoembryonic antigen (CEA) describes a set of highly-related glycoproteins involved in cell adhesion. CEA is normally produced in gastrointestinal tissue during fetal development, but the production stops before birth. Consequently, CEA is usually present at very low levels in the blood of healthy adults. However, the serum levels are raised in some types of cancer, which means that it can be used as a tumor marker in clinical tests. Serum levels can also be elevated in heavy smokers.
Immunoprecipitation (IP) is the technique of precipitating a protein antigen out of solution using an antibody that specifically binds to that particular protein. This process can be used to isolate and concentrate a particular protein from a sample containing many thousands of different proteins. Immunoprecipitation requires that the antibody be coupled to a solid substrate at some point in the procedure.

A serotype or serovar is a distinct variation within a species of bacteria or virus or among immune cells of different individuals. These microorganisms, viruses, or cells are classified together based on their surface antigens, allowing the epidemiologic classification of organisms to a level below the species. A group of serovars with common antigens is called a serogroup or sometimes serocomplex.

Immunoelectrophoresis is a general name for a number of biochemical methods for separation and characterization of proteins based on electrophoresis and reaction with antibodies. All variants of immunoelectrophoresis require immunoglobulins, also known as antibodies, reacting with the proteins to be separated or characterized. The methods were developed and used extensively during the second half of the 20th century. In somewhat chronological order: Immunoelectrophoretic analysis, crossed immunoelectrophoresis, rocket-immunoelectrophoresis, fused rocket immunoelectrophoresis ad modum Svendsen and Harboe, affinity immunoelectrophoresis ad modum Bøg-Hansen.

Ouchterlony double immunodiffusion is an immunological technique used in the detection, identification and quantification of antibodies and antigens, such as immunoglobulins and extractable nuclear antigens. The technique is named after Örjan Ouchterlony, the Swedish physician who developed the test in 1948 to evaluate the production of diphtheria toxins from isolated bacteria.

p-ANCA, or MPO-ANCA, or perinuclear anti-neutrophil cytoplasmic antibodies, are antibodies that stain the material around the nucleus of a neutrophil. They are a special class of anti-neutrophil cytoplasmic antibodies.
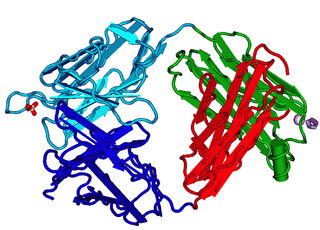
The fragment antigen-binding region is a region on an antibody that binds to antigens. It is composed of one constant and one variable domain of each of the heavy and the light chain. The variable domain contains the paratope, comprising a set of complementarity-determining regions, at the amino terminal end of the monomer. Each arm of the Y thus binds an epitope on the antigen.
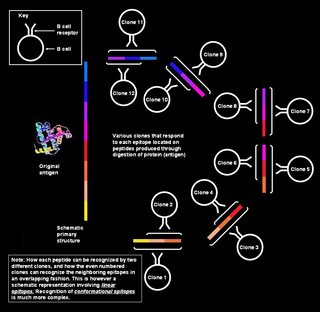
Polyclonal B cell response is a natural mode of immune response exhibited by the adaptive immune system of mammals. It ensures that a single antigen is recognized and attacked through its overlapping parts, called epitopes, by multiple clones of B cell.

Immunofixation permits the detection and typing of monoclonal antibodies or immunoglobulins in serum or urine. It is of great importance for the diagnosis and monitoring of certain blood related diseases such as myeloma.
Örjan Thomas Ouchterlony was a Swedish bacteriologist and immunologist who is credited with the creation of the Ouchterlony double immuno diffusion test in the 1940s. He was trained at Karolinska Institute, where his received his medical doctorate. He worked at Sweden's State Bacteriology Laboratory from 1935 to 1952. Ouchterlony was a professor of bacteriology at the Medical Faculty of Gothenburg University from 1952 to 1980 and was elected a member of the Royal Swedish Academy of Sciences in 1968. In addition to his laboratory work, he did research in field epidemiology of infectious diseases and worked and lectured in Africa and the United States, as well as in several countries in Europe. Upon his retirement in 1980, the successor to his professorial chair was Jan Holmgren.
The Ouchterlony plate is one of the more frequently used techniques for the identification of antigens and antibodies, by measurement of diffusion gradients in gel. Among its many applications has been the search for tumor-specific antigens. The technique was introduced by Örjan Ouchterlony of Sweden, in 1948, initially for the in vitro testing of the toxin-producing capacity of diphtheria bacteria. The technique was proved well suited to the analysis of complex serological systems, including analysis that helped to elucidate the structural heterogeneity of immunoglobulins. Ouchterlony reviewed the history of the developments, which extends back to the late Nineteenth Century.
A neutralizing antibody (NAb) is an antibody that defends a cell from a pathogen or infectious particle by neutralizing any effect it has biologically. Neutralization renders the particle no longer infectious or pathogenic. Neutralizing antibodies are part of the humoral response of the adaptive immune system against viruses, intracellular bacteria and microbial toxin. By binding specifically to surface structures (antigen) on an infectious particle, neutralizing antibodies prevent the particle from interacting with its host cells it might infect and destroy.
Cell CANARY is a recent technology that uses genetically engineered B cells to identify pathogens. Existing pathogen detection technologies include the Integrated Biological Detection System and the Joint Chemical Agent Detector.
Antigen-antibody interaction, or antigen-antibody reaction, is a specific chemical interaction between antibodies produced by B cells of the white blood cells and antigens during immune reaction. The antigens and antibodies combine by a process called agglutination. It is the fundamental reaction in the body by which the body is protected from complex foreign molecules, such as pathogens and their chemical toxins. In the blood, the antigens are specifically and with high affinity bound by antibodies to form an antigen-antibody complex. The immune complex is then transported to cellular systems where it can be destroyed or deactivated.

Blood compatibility testing is conducted in a medical laboratory to identify potential incompatibilities between blood group systems in blood transfusion. It is also used to diagnose and prevent some complications of pregnancy that can occur when the baby has a different blood group from the mother. Blood compatibility testing includes blood typing, which detects the antigens on red blood cells that determine a person's blood type; testing for unexpected antibodies against blood group antigens ; and, in the case of blood transfusions, mixing the recipient's plasma with the donor's red blood cells to detect incompatibilities (crossmatching). Routine blood typing involves determining the ABO and RhD type, and involves both identification of ABO antigens on red blood cells and identification of ABO antibodies in the plasma. Other blood group antigens may be tested for in specific clinical situations.













