Related Research Articles

The plum pudding model was the first scientific model of the atom with internal structure. It was first proposed by J. J. Thomson in 1904 following his discovery of the electron in 1897, but it was subsequently rendered obsolete by Ernest Rutherford's discovery of the atomic nucleus in 1911. The model tried to account for two properties of atoms then known: that there are electrons and that atoms have no net electric charge. Logically there had to be an equal amount of positive charge to balance out the negative charge of the electrons. As he had no idea of the source of this positive charge, Thomson tentatively proposed that it was everywhere in the atom, the atom being in the shape of a sphere for the sake of mathematical simplicity. Following from this, Thomson imagined that the balance of electrostatic forces in the atom would distribute the electrons more or less evenly throughout this hypothetical sphere.

In physics, electromagnetism is an interaction that occurs between particles with electric charge via electromagnetic fields. The electromagnetic force is one of the four fundamental forces of nature. It is the dominant force in the interactions of atoms and molecules. Electromagnetism can be thought of as a combination of electrostatics and magnetism, which are distinct but closely intertwined phenomena. Electromagnetic forces occur between any two charged particles. Electric forces cause an attraction between particles with opposite charges and repulsion between particles with the same charge, while magnetism is an interaction that occurs between charged particles in relative motion. These two forces are described in terms of electromagnetic fields. Macroscopic charged objects are described in terms of Coulomb's law for electricity and Ampère's force law for magnetism; the Lorentz force describes microscopic charged particles.

Electric charge is the physical property of matter that causes it to experience a force when placed in an electromagnetic field. Electric charge can be positive or negative. Like charges repel each other and unlike charges attract each other. An object with no net charge is referred to as electrically neutral. Early knowledge of how charged substances interact is now called classical electrodynamics, and is still accurate for problems that do not require consideration of quantum effects.
In physics, the fundamental interactions or fundamental forces are the interactions that do not appear to be reducible to more basic interactions. There are four fundamental interactions known to exist in nature:

Magnetism is the class of physical attributes that occur through a magnetic field, which allows objects to attract or repel each other. Because both electric currents and magnetic moments of elementary particles give rise to a magnetic field, magnetism is one of two aspects of electromagnetism.
The caloric theory is an obsolete scientific theory that heat consists of a self-repellent fluid called caloric that flows from hotter bodies to colder bodies. Caloric was also thought of as a weightless gas that could pass in and out of pores in solids and liquids. The "caloric theory" was superseded by the mid-19th century in favor of the mechanical theory of heat, but nevertheless persisted in some scientific literature—particularly in more popular treatments—until the end of the 19th century.
In physics, action at a distance is the concept that an object's motion can be affected by another object without being in physical contact with it; that is, the non-local interaction of objects that are separated in space. Coulomb's law and Newton's law of universal gravitation are based on action at a distance.

The nuclear force is a force that acts between hadrons, most commonly observed between protons and neutrons of atoms. Neutrons and protons, both nucleons, are affected by the nuclear force almost identically. Since protons have charge +1 e, they experience an electric force that tends to push them apart, but at short range the attractive nuclear force is strong enough to overcome the electrostatic force. The nuclear force binds nucleons into atomic nuclei.

The history of thermodynamics is a fundamental strand in the history of physics, the history of chemistry, and the history of science in general. Due to the relevance of thermodynamics in much of science and technology, its history is finely woven with the developments of classical mechanics, quantum mechanics, magnetism, and chemical kinetics, to more distant applied fields such as meteorology, information theory, and biology (physiology), and to technological developments such as the steam engine, internal combustion engine, cryogenics and electricity generation. The development of thermodynamics both drove and was driven by atomic theory. It also, albeit in a subtle manner, motivated new directions in probability and statistics; see, for example, the timeline of thermodynamics.
Fluid theories of electricity are outdated theories that postulated one or more electrical fluids which were thought to be responsible for many electrical phenomena in the history of electromagnetism. The "two-fluid" theory of electricity, created by Charles François de Cisternay du Fay, postulated that electricity was the interaction between two electrical 'fluids.' An alternate simpler theory was proposed by Benjamin Franklin, called the unitary, or one-fluid, theory of electricity. This theory claimed that electricity was really one fluid, which could be present in excess, or absent from a body, thus explaining its electrical charge. Franklin's theory explained how charges could be dispelled and how they could be passed through a chain of people. The fluid theories of electricity eventually became updated to include the effects of magnetism, and electrons.

Nuclear binding energy in experimental physics is the minimum energy that is required to disassemble the nucleus of an atom into its constituent protons and neutrons, known collectively as nucleons. The binding energy for stable nuclei is always a positive number, as the nucleus must gain energy for the nucleons to move apart from each other. Nucleons are attracted to each other by the strong nuclear force. In theoretical nuclear physics, the nuclear binding energy is considered a negative number. In this context it represents the energy of the nucleus relative to the energy of the constituent nucleons when they are infinitely far apart. Both the experimental and theoretical views are equivalent, with slightly different emphasis on what the binding energy means.
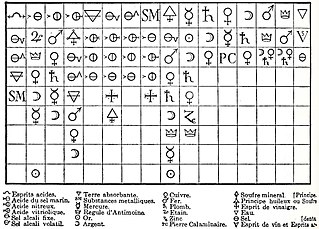
In the history of chemistry, the chemical revolution, also called the first chemical revolution, was the reformulation of chemistry during the seventeenth and eighteenth centuries, which culminated in the law of conservation of mass and the oxygen theory of combustion.
Electrochemistry, a branch of chemistry, went through several changes during its evolution from early principles related to magnets in the early 16th and 17th centuries, to complex theories involving conductivity, electric charge and mathematical methods. The term electrochemistry was used to describe electrical phenomena in the late 19th and 20th centuries. In recent decades, electrochemistry has become an area of current research, including research in batteries and fuel cells, preventing corrosion of metals, the use of electrochemical cells to remove refractory organics and similar contaminants in wastewater electrocoagulation and improving techniques in refining chemicals with electrolysis and electrophoresis.

The history of electromagnetic theory begins with ancient measures to understand atmospheric electricity, in particular lightning. People then had little understanding of electricity, and were unable to explain the phenomena. Scientific understanding and research into the nature of electricity grew throughout the eighteenth and nineteenth centuries through the work of researchers such as André-Marie Ampère, Charles-Augustin de Coulomb, Michael Faraday, Carl Friedrich Gauss and James Clerk Maxwell.
A stress field is the distribution of internal forces in a body that balance a given set of external forces. Stress fields are widely used in fluid dynamics and materials science. Consider that one can picture the stress fields as the stress created by adding an extra half plane of atoms to a crystal. The bonds are clearly stretched around the location of the dislocation and this stretching causes the stress field to form. Atomic bonds farther and farther away from the dislocation centre are less and less stretched which is why the stress field dissipates as the distance from the dislocation centre increases. Each dislocation within the material has a stress field associated with it. The creation of these stress fields is a result of the material trying to dissipate mechanical energy that is being exerted on the material. By convention, these dislocations are labelled as either positive or negative depending on whether the stress field of the dislocation is mostly compressive or tensile.

Gas is one of the four fundamental states of matter. The others are solid, liquid, and plasma. A pure gas may be made up of individual atoms, elemental molecules made from one type of atom, or compound molecules made from a variety of atoms. A gas mixture, such as air, contains a variety of pure gases. What distinguishes gases from liquids and solids is the vast separation of the individual gas particles. This separation usually makes a colorless gas invisible to the human observer.
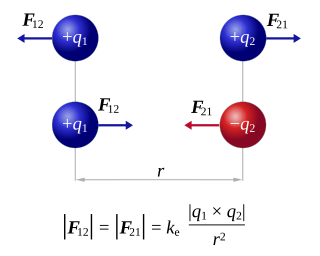
Coulomb's inverse-square law, or simply Coulomb's law, is an experimental law of physics that calculates the amount of force between two electrically charged particles at rest. This electric force is conventionally called the electrostatic force or Coulomb force. Although the law was known earlier, it was first published in 1785 by French physicist Charles-Augustin de Coulomb. Coulomb's law was essential to the development of the theory of electromagnetism and maybe even its starting point, as it allowed meaningful discussions of the amount of electric charge in a particle.
Chemistry: A Volatile History is a 2010 BBC documentary on the history of chemistry presented by Jim Al-Khalili. It was nominated for the 2010 British Academy Television Awards in the category Specialist Factual.
Edward Peart was an English physician.
References
- ↑ Textbook of Human Physiology, Leonard Landois , William Stirling, 1889
- ↑ Scientific Realism: How Science Tracks Truth, Stathis Psillos, 1999, Routledge
- ↑ Jenkin, Fleeming (1868). "The Atomic Theory of Lucretius". The North British Review. 48: 239. Retrieved 30 May 2014.
- ↑ Time of describing a given space from rest under the action of a force varying as the distance from a fixed point. Principia By Sir Isaac Newton. Pg., 86
- ↑ As defined by Boscovich and the French School, an atom was no longer a substantial entity, but a mathematical point, a center of force, and "matter" is a crowd of such points, endowed with inertia and powers of attraction and repulsion.(The Monist: Volume 20. By Edward C. Hegeler, Paul Carus, Hegeler Institute, 1910. Page 220.)
- ↑ The North British review. (1868). Edinburgh: W.P. Kennedy Pg 126.
- ↑ J Lardner, D. (1853 ). A hand-book of mechanics. Philadelphia 223.
- ↑ U.S. patent 60986, Improved imponderable fluid, and mode of generating the same. Jan 1, 1867.
- ↑ Haydn, J., & In Vincent, B. (1893). Haydn's dictionary of dates and universal information relating to all ages and nations. N.Y: G.P. Putnam Pg 28