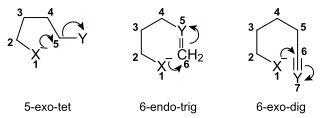Nucleotides are organic molecules composed of a nitrogenous base, a pentose sugar and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules within all life-forms on Earth. Nucleotides are obtained in the diet and are also synthesized from common nutrients by the liver.
The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring. Friedel–Crafts reactions are of two main types: alkylation reactions and acylation reactions. Both proceed by electrophilic aromatic substitution.

Imidazole (ImH) is an organic compound with the formula (CH)3(NH)N. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. It can be classified as a heterocycle, specifically as a diazole.
The Fischer oxazole synthesis is a chemical synthesis of an oxazole from a cyanohydrin and an aldehyde in the presence of anhydrous hydrochloric acid. This method was discovered by Emil Fischer in 1896. The cyanohydrin itself is derived from a separate aldehyde. The reactants of the oxazole synthesis itself, the cyanohydrin of an aldehyde and the other aldehyde itself, are usually present in equimolar amounts. Both reactants usually have an aromatic group, which appear at specific positions on the resulting heterocycle.
Acyloin condensation is a reductive coupling of two carboxylic esters using impure metallic sodium to yield an α-hydroxyketone, also known as an acyloin.
The Pictet–Spengler reaction is a chemical reaction in which a β-arylethylamine undergoes condensation with an aldehyde or ketone followed by ring closure. The reaction was first discovered in 1911 by Amé Pictet and Theodor Spengler. Traditionally, an acidic catalyst in protic solvent was employed with heating; however, the reaction has been shown to work in aprotic media in superior yields and sometimes without acid catalysis. The Pictet–Spengler reaction can be considered a special case of the Mannich reaction, which follows a similar reaction pathway. The driving force for this reaction is the electrophilicity of the iminium ion generated from the condensation of the aldehyde and amine under acid conditions. This explains the need for an acid catalyst in most cases, as the imine is not electrophilic enough for ring closure but the iminium ion is capable of undergoing the reaction.
The Reissert indole synthesis is a series of chemical reactions designed to synthesize indole or substituted-indoles from ortho-nitrotoluene 1 and diethyl oxalate 2.

Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry.
Dioxolane is a heterocyclic acetal with the chemical formula (CH2)2O2CH2. It is related to tetrahydrofuran (THF) by replacement of the methylene group (CH2) at the 2-position with an oxygen atom. The corresponding saturated 6-membered C4O2 rings are called dioxanes. The isomeric 1,2-dioxolane (wherein the two oxygen centers are adjacent) is a peroxide. 1,3-dioxolane is used as a solvent and as a comonomer in polyacetals.
Ring-closing metathesis (RCM) is a widely used variation of olefin metathesis in organic chemistry for the synthesis of various unsaturated rings via the intramolecular metathesis of two terminal alkenes, which forms the cycloalkene as the E- or Z- isomers and volatile ethylene.
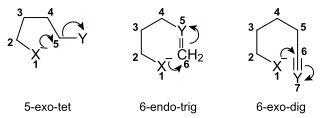
Baldwin's rules in organic chemistry are a series of guidelines outlining the relative favorabilities of ring closure reactions in alicyclic compounds. They were first proposed by Jack Baldwin in 1976.

Mesembrine is an alkaloid primarily derived from the plant Sceletium tortuosum, commonly known as kanna. This compound is noted for its psychoactive properties, particularly as a serotonin reuptake inhibitor, which contributes to its potential use in treating mood disorders and anxiety. Mesembrine has garnered interest in both traditional medicine and modern pharmacology, where it is explored for its effects on enhancing mood and cognitive function. The plant itself has a long history of use by indigenous peoples in southern Africa, who utilized it for its mood-enhancing and stress-relieving effects, often consuming it in various forms such as teas or chews.
Iodolactonization is an organic reaction that forms a ring by the addition of an oxygen and iodine across a carbon-carbon double bond. It is an intramolecular variant of the halohydrin synthesis reaction. The reaction was first reported by M. J. Bougalt in 1904 and has since become one of the most effective ways to synthesize lactones. Strengths of the reaction include the mild conditions and incorporation of the versatile iodine atom into the product.

Indole is an organic compound with the formula C6H4CCNH3. Indole is classified as an aromatic heterocycle. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indoles are derivatives of indole where one or more of the hydrogen atoms have been replaced by substituent groups. Indoles are widely distributed in nature, most notably as amino acid tryptophan and neurotransmitter serotonin.

Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical, designer, and experimental drugs.

Oxazoline is a five-membered heterocyclic organic compound with the formula C3H5NO. It is the parent of a family of compounds called oxazolines, which contain non-hydrogenic substituents on carbon and/or nitrogen. Oxazolines are the unsaturated analogues of oxazolidines, and they are isomeric with isoxazolines, where the N and O are directly bonded. Two isomers of oxazoline are known, depending on the location of the double bond.
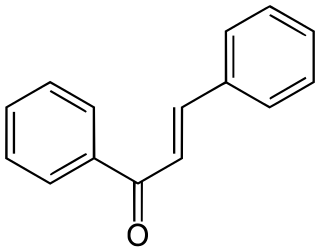
Chalconoids, also known as chalcones, are natural phenols derived from chalcone. They form the central core for a variety of important biological compounds.
The Buchner ring expansion is a two-step organic C-C bond forming reaction used to access 7-membered rings. The first step involves formation of a carbene from ethyl diazoacetate, which cyclopropanates an aromatic ring. The ring expansion occurs in the second step, with an electrocyclic reaction opening the cyclopropane ring to form the 7-membered ring.
Rearrangements, especially those that can participate in cascade reactions, such as the aza-Cope rearrangements, are of high practical as well as conceptual importance in organic chemistry, due to their ability to quickly build structural complexity out of simple starting materials. The aza-Cope rearrangements are examples of heteroatom versions of the Cope rearrangement, which is a [3,3]-sigmatropic rearrangement that shifts single and double bonds between two allylic components. In accordance with the Woodward-Hoffman rules, thermal aza-Cope rearrangements proceed suprafacially. Aza-Cope rearrangements are generally classified by the position of the nitrogen in the molecule :
The Cadogan–Sundberg indole synthesis, or simply Cadogan indole synthesis, is a name reaction in organic chemistry that allows for the generation of indoles from o-nitrostyrenes with the use of trialkyl phosphites, such as triethyl phosphite.