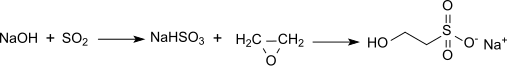| |
 Isethionic acid | |
| Names | |
|---|---|
| Preferred IUPAC name 2-Hydroxyethane-1-sulfonic acid | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.003.169 |
| KEGG | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C2H6O4S | |
| Molar mass | 126.13 g/mol |
| Density | 1.63 g/cm3 |
| Acidity (pKa) | 1.39 (predicted) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Isethionic acid is an organosulfur compound containing an alkyl sulfonic acid located beta to a hydroxy group. Its discovery is generally attributed to Heinrich Gustav Magnus, who prepared it by the action of solid sulfur trioxide on ethanol in 1833. [1] It is a white water-soluble solid used in the manufacture of certain surfactants and in the industrial production of taurine. It is most commonly available in the form of its sodium salt (sodium isethionate).