
In organic chemistry, a ketone is an organic compound with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens. Halides are also commonly introduced using salts of the halides and halogen acids. Many specialized reagents exist for and introducing halogens into diverse substrates, e.g. thionyl chloride.
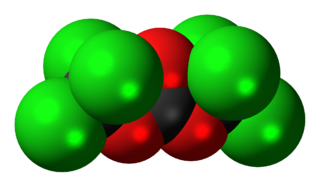
Triphosgene (bis(trichloromethyl) carbonate (BTC) is a chemical compound with the formula OC(OCCl3)2. It is used as a solid substitute for phosgene, which is a gas and diphosgene, which is a liquid. Triphosgene is stable up to 200 °C. Triphosgene is used in a variety of halogenation reactions.

Trimethylsilyldiazomethane is the organosilicon compound with the formula (CH3)3SiCHN2. It is classified as a diazo compound. Trimethylsilyldiazomethane is a commercially available reagent used in organic chemistry as a methylating agent and as a source of CH2 group. Its behavior is akin to the less convenient reagent diazomethane.
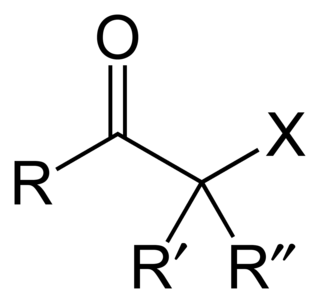
In organic chemistry, an α-halo ketone is a functional group consisting of a ketone group or more generally a carbonyl group with an α-halogen substituent. α-Halo ketones are alkylating agents. Prominent α-halo ketones include phenacyl bromide and chloroacetone.

Tebbe's reagent is the organometallic compound with the formula (C5H5)2TiCH2ClAl(CH3)2. It is used in the methylidenation of carbonyl compounds, that is it converts organic compounds containing the R2C=O group into the related R2C=CH2 derivative. It is a red solid that is pyrophoric in the air, and thus is typically handled with air-free techniques. It was originally synthesized by Fred Tebbe at DuPont Central Research.
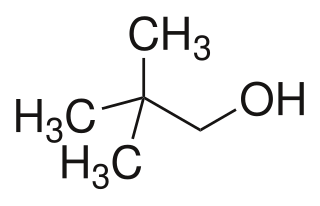
Neopentyl alcohol is a compound with formula (CH3)3CCH2OH. It is a colorless solid. The compound is one of the eight isomers of pentyl alcohol.
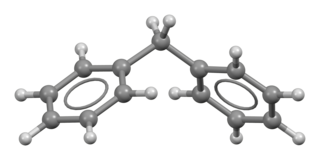
Diphenylmethane is an organic compound with the formula (C6H5)2CH2 (often abbreviated CH
2Ph
2). The compound consists of methane wherein two hydrogen atoms are replaced by two phenyl groups. It is a white solid.
Pivalic acid is a carboxylic acid with a molecular formula of (CH3)3CCO2H. This colourless, odiferous organic compound is solid at room temperature. Two abbreviation for pivalic acid are t-BuC(O)OH and PivOH. The pivalyl or pivaloyl group is abbreviated t-BuC(O).

Trimethylsilyl azide is the organosilicon compound with the formula (CH3)3SiN3. A colorless liquid, it is a reagent in organic chemistry, serving as the equivalent of hydrazoic acid.

Trimethyltin chloride is an organotin compound with the formula (CH3)3SnCl. It is a white solid that is highly toxic and malodorous. It is susceptible to hydrolysis.
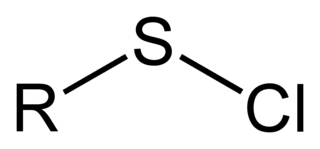
In organosulfur chemistry, a sulfenyl chloride is a functional group with the connectivity R−S−Cl, where R is alkyl or aryl. Sulfenyl chlorides are reactive compounds that behave as sources of RS+. They are used in the formation of RS−N and RS−O bonds. According to IUPAC nomenclature they are named as alkyl thiohypochlorites, i.e. esters of thiohypochlorous acid.
Tridecylic acid, or tridecanoic acid, is the organic compound with the formula CH3(CH2)11CO2H. It is a 13-carbon saturated fatty acid. It is a white solid.

Propargyl bromide, also known as 3-bromo-prop-1-yne, is an organic compound with the chemical formula HC≡CCH2Br. A colorless liquid, it is a halogenated organic compound consisting of propyne with a bromine substituent on the methyl group. It has a lachrymatory effect, like related compounds. The compound is used as a reagent in organic synthesis.
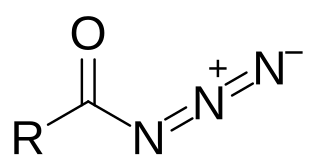
Acyl azides are carboxylic acid derivatives with the general formula RCON3. These compounds, which are a subclass of organic azides, are generally colorless.
Heneicosylic acid, or heneicosanoic acid, is the organic compound with the formula CH3(CH2)19CO2H. It is the straight-chain 21-carbon saturated fatty acid. It is a colorless solid.

tert-Butyldimethylsilyl chloride is an organosilicon compound with the formula (Me3C)Me2SiCl (Me = CH3). It is commonly abbreviated as TBSCl or TBDMSCl. It is a chlorosilane containing two methyl groups and a tert-butyl group. As such it is more bulky that trimethylsilyl chloride. It is a colorless or white solid that is soluble in many organic solvents but reacts with water and alcohols. The compound is used to protect alcohols in organic synthesis.
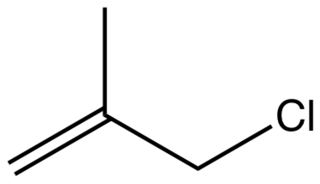
Methallyl chloride is the organic compound with the formula CH2=C(CH3)CH2Cl. It is a colorless liquid and a lacrymator. Its properties are similar to those of allyl chloride. It is a strong alkylating agent used to install isobutenyl groups.

3,5-Dimethylpyrazole is an organic compound with the formula (CH3C)2CHN2H. It is one of several isomeric derivatives of pyrazole that contain two methyl substituents. The compound is unsymmetrical but the corresponding conjugate acid (pyrazolium) and conjugate base (pyrazolide) have C2v symmetry. It is a white solid that dissolves well in polar organic solvents.
Hydroxymethylation is a chemical reaction that installs the CH2OH group. The transformation can be implemented in many ways and applies to both industrial and biochemical processes.

















