Sodium laureth sulfate (SLES), an accepted contraction of sodium lauryl ether sulfate (SLES), also called sodium alkylethersulfate, is an anionic detergent and surfactant found in many personal care products and for industrial uses. SLES is an inexpensive and very effective foaming agent. SLES, sodium lauryl sulfate (SLS), ammonium lauryl sulfate (ALS), and sodium pareth sulfate are surfactants that are used in many cosmetic products for their cleaning and emulsifying properties. It is derived from palm kernel oil or coconut oil. In herbicides, it is used as a surfactant to improve absorption of the herbicidal chemicals and reduces time the product takes to be rainfast, when enough of the herbicidal agent will be absorbed.
Sodium dodecyl sulfate (SDS) or sodium lauryl sulfate (SLS), sometimes written sodium laurilsulfate, is an organic compound with the formula CH3(CH2)11OSO3Na and structure H3C−(CH2)11−O−S(=O)2−O−Na+. It is an anionic surfactant used in many cleaning and hygiene products. This compound is the sodium salt of the 12-carbon organosulfate. Its hydrocarbon tail combined with a polar "headgroup" give the compound amphiphilic properties that make it useful as a detergent. SDS is also component of mixtures produced from inexpensive coconut and palm oils. SDS is a common component of many domestic cleaning, personal hygiene and cosmetic, pharmaceutical, and food products, as well as of industrial and commercial cleaning and product formulations.

Surfactants are chemical compounds that decrease the surface tension or interfacial tension between two liquids, a liquid and a gas, or a liquid and a solid. The word "surfactant" is a blend of surface-active agent, coined c. 1950. As they consist of a water-repellent and a water-attracting part, they enable water and oil to mix; they can form foam and facilitate the detachment of dirt.

Vitis vinifera, the common grape vine, is a species of flowering plant, native to the Mediterranean region, Central Europe, and southwestern Asia, from Morocco and Portugal north to southern Germany and east to northern Iran. There are currently between 5,000 and 10,000 varieties of Vitis vinifera grapes though only a few are of commercial significance for wine and table grape production.
Ammonium lauryl sulfate (ALS) is the common name for ammonium dodecyl sulfate (CH3(CH2)10CH2OSO3NH4). The anion consists of a nonpolar hydrocarbon chain and a polar sulfate end group. The combination of nonpolar and polar groups confers surfactant properties to the anion: it facilitates dissolution of both polar and non-polar materials. This salt is classified as a sulfate ester. It is made from coconut or palm kernel oil for use primarily in shampoos and body-wash as a foaming agent. Lauryl sulfates are very high-foam surfactants that disrupt the surface tension of water in part by forming micelles at the surface-air interface.
Lauryl tryptose broth (LTB) is a selective growth medium (broth) for coliforms.
Dodecanol, or lauryl alcohol, is an organic compound produced industrially from palm kernel oil or coconut oil. It is a fatty alcohol. Sulfate esters of lauryl alcohol, especially sodium lauryl sulfate, are very widely used as surfactants. Sodium lauryl sulfate and the related dodecanol derivatives ammonium lauryl sulfate and sodium laureth sulfate are all used in shampoos. Dodecanol is tasteless, colorless, and has a floral odor.

Apterin is a furanocoumarin and the glucoside of vaginol. It has been isolated from the root of plants in the family Apiaceae such as members of the genus Angelica, including the garden angelica and Zizia aptera.
Decyl glucoside is a mild non-ionic surfactant used in cosmetic formularies, including baby shampoo and in products for individuals with a sensitive skin. Many natural personal care companies use this cleanser because it is plant-derived, biodegradable, and gentle for all hair types.

Lauryl methyl gluceth-10 hydroxypropyl dimonium chloride is an ingredient in some types of soaps and personal care products. It is used as a substantive conditioning humectant. This chemical is a type of methyl glucoside derivative, which has been modified by ethoxylation and quaternization. A synthetic pathway for lauryl methyl gluceth-10 hydroxypropyldimonium chloride and other methyl glucoside humectants has been outlined in trade literature.

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.
Octyl glucoside is a nonionic surfactant frequently used to solubilise integral membrane proteins for studies in biochemistry. Structurally, it is a glycoside derived from glucose and octanol. Like Genapol X-100 and Triton X-100, it is a nonphysiological amphiphile that makes lipid bilayers less "stiff".

Isorhamnetin is an O-methylated flavon-ol from the class of flavonoids. A common food source of this 3'-methoxylated derivative of quercetin and its glucoside conjugates are pungent yellow or red onions, in which it is a minor pigment, quercetin-3,4'-diglucoside and quercetin-4'-glucoside and the aglycone quercetin being the major pigments. Pears, olive oil, wine and tomato sauce are rich in isorhamnetin. Almond skin is a rich source of isorhamnetin-3-O-rutinoside and isorhamnetin-3-O-glucoside, in some cultivars they comprise 75% of the polyphenol content, the total of which can exceed 10 mg/100 gram almond. Others sources include the spice, herbal medicinal and psychoactive Mexican tarragon (Tagetes lucida), which is described as accumulating isorhamnetin and its 7-O-glucoside derivate. Nopal is also a good source of isorhamnetin, which can be extracted by supercritical fluid extraction assisted by enzymes.

Biochanin A is an O-methylated isoflavone. It is a natural organic compound in the class of phytochemicals known as flavonoids. Biochanin A can be found in red clover in soy, in alfalfa sprouts, in peanuts, in chickpea and in other legumes.

Tectoridin is an isoflavone, a type of flavonoid. It is the 7-glucoside of tectorigenin and can be isolated from flowers of Pueraria thunbergiana (Leguminosae).
Potassium lauryl sulfate a detergent similar to sodium lauryl sulfate. Potassium lauryl sulfate is used in mud soaps. It has a better cleansing action indicated by the presence of the potassium ion.
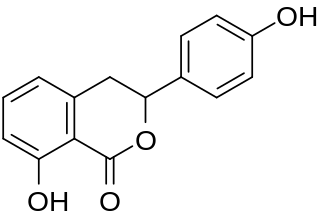
Hydrangenol is a dihydroisocoumarin. It can be found in Hydrangea macrophylla, as well as its 8-O-glucoside. (−)-Hydrangenol 4′-O-glucoside and (+)-hydrangenol 4′-O-glucoside can be found in Hydrangeae Dulcis Folium, the processed leaves of H. macrophylla var. thunbergii.

Kaempferol 7-O-glucoside is a flavonol glucoside. It can be found in Smilax china, and in the fern Asplenium rhizophyllum, and its hybrid descendants, as part of a complex with caffeic acid.

Delphinidin 3-O-(6-p-coumaroyl)glucoside is a p-coumaroylated anthocyanin. It can be found in some red Vitis vinifera grape cultivars and in red wine.

p-Coumaric acid glucoside is a hydroxycinnamic acid, an organic compound found in commercial breads containing flaxseed.













