| Legume lectin domain (or L-type lectin domain) | |
|---|---|
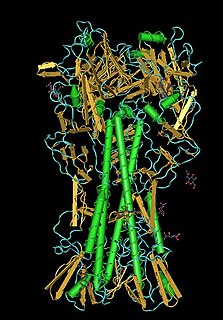
 Structure of the monosaccharide binding site of lentil lectin. [1] | |
| Identifiers | |
| Symbol | Lectin_legB |
| Pfam | PF00139 |
| Pfam clan | CL0004 |
| InterPro | IPR001220 |
| PROSITE | PDOC00278 |
| SCOP2 | 1lem / SCOPe / SUPFAM |
The legume lectins (or L-type lectins) are a family of sugar-binding proteins or lectins found in the seeds and, in smaller amounts, in the roots, stems, leaves and bark of plants of the family Fabaceae. [2] [3] The exact function of the legume lectins in vivo is unknown but they are probably involved in the defense of plants against predators. Related proteins in other plant families and in animals have also been found. They have been used for decades as a model system for the study of protein-carbohydrate interactions, because they show an amazing variety of binding specificities and are easy to obtain and purify. Over the years, a quite impressive amount of structural data has been gathered. [3] Well-studied members of this protein family include phytohemagglutinin and concanavalin A.