
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.

The Pauson–Khand (PK) reaction is a chemical reaction, described as a [2+2+1] cycloaddition. In it, an alkyne, an alkene and carbon monoxide combine into a α,β-cyclopentenone in the presence of a metal-carbonyl catalyst.

The Corey–Itsuno reduction, also known as the Corey–Bakshi–Shibata (CBS) reduction, is a chemical reaction in which a prochiral ketone is enantioselectively reduced to produce the corresponding chiral, non-racemic alcohol. The oxazaborolidine reagent which mediates the enantioselective reduction of ketones was previously developed by the laboratory of Itsuno and thus this transformation may more properly be called the Itsuno-Corey oxazaborolidine reduction.
The Overman rearrangement is a chemical reaction that can be described as a Claisen rearrangement of allylic alcohols to give allylic trichloroacetamides through an imidate intermediate. The Overman rearrangement was discovered in 1974 by Larry Overman.
The Reformatsky reaction is an organic reaction which condenses aldehydes or ketones with α-halo esters using metallic zinc to form β-hydroxy-esters:
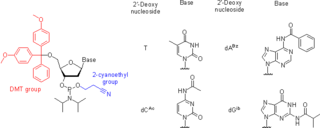
Nucleoside phosphoramidites are derivatives of natural or synthetic nucleosides. They are used to synthesize oligonucleotides, relatively short fragments of nucleic acid and their analogs. Nucleoside phosphoramidites were first introduced in 1981 by Beaucage and Caruthers. To avoid undesired side reactions, reactive hydroxy and exocyclic amino groups present in natural or synthetic nucleosides are appropriately protected. As long as a nucleoside analog contains at least one hydroxy group, the use of the appropriate protecting strategy allows one to convert that to the respective phosphoramidite and to incorporate the latter into synthetic nucleic acids. To be incorporated in the middle of an oligonucleotide chain using phosphoramidite strategy, the nucleoside analog must possess two hydroxy groups or, less often, a hydroxy group and another nucleophilic group (amino or mercapto). Examples include, but are not limited to, alternative nucleotides, LNA, morpholino, nucleosides modified at the 2'-position (OMe, protected NH2, F), nucleosides containing non-canonical bases (hypoxanthine and xanthine contained in natural nucleosides inosine and xanthosine, respectively, tricyclic bases such as G-clamp, etc.) or bases derivatized with a fluorescent group or a linker arm.
The reduction of nitro compounds are chemical reactions of wide interest in organic chemistry. The conversion can be effected by many reagents. The nitro group was one of the first functional groups to be reduced. Alkyl and aryl nitro compounds behave differently. Most useful is the reduction of aryl nitro compounds.
The Fukuyama coupling is a coupling reaction taking place between a thioester and an organozinc halide in the presence of a palladium catalyst. The reaction product is a ketone. This reaction was discovered by Tohru Fukuyama et al. in 1998.

Michael E. Jung is a Professor of Chemistry in the Department of Chemistry and Biochemistry at the University of California at Los Angeles.

Zinc trifluoromethanesulfonate or zinc triflate is the zinc salt of trifluoromethanesulfonic acid. It is commonly used as a Lewis acid catalyst, e.g. in silylations.

Protopanaxadiol (PPD) is an organic compound that is an aglycone of ginsenosides, a group of steroid glycosides. It is a dammarane-type tetracyclic terpene sapogenin found in ginseng and in notoginseng.
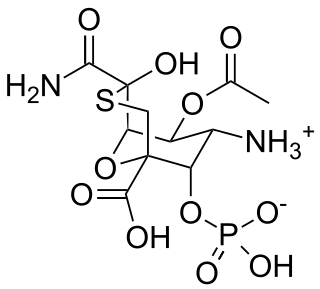
Tagetitoxin (TGT) is a bacterial phytotoxin produced by Pseudomonas syringae pv. tagetis.
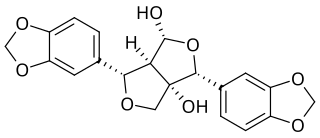
Gummadiol is a lignan hemiacetal. It can be isolated from the heartwood of Gmelina arborea.

Lavendamycin is a naturally occurring chemical compound discovered in fermentation broth of the soil bacterium Streptomyces lavendulae. Lavendamycin has antibiotic properties and anti-proliferative effects against several cancer cell lines. The use of lavendamycin as a cytotoxic agent in cancer therapy failed due to poor water solubility and non-specific cytotoxicity. The study of lavendamycin-based analogs designed to overcome these liabilities has been an area of research.
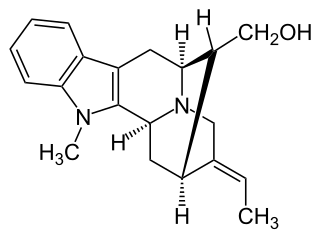
Affinisine is a monoterpenoid indole alkaloid which can be isolated from plants of the genus Tabernaemontana. Structurally, it can be considered a member of the sarpagine alkaloid family and may be synthesized from tryptophan via a Pictet-Spengler reaction.
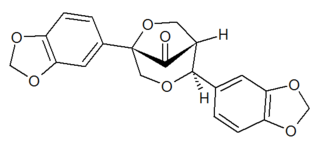
Gmelanone is a lignan found in the heartwood of Gmelina arborea. Arboreol can be transformed by acid catalysis into gmelanone.
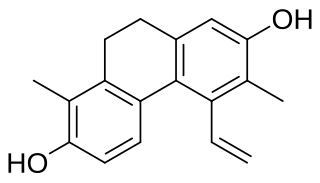
Juncusol is a 9,10-dihydrophrenathrene found in Juncus species such as J. acutus, J. effusus or J. roemerianus.
In organic chemistry, the Davis oxidation or Davis' oxaziridine oxidation refers to oxidations involving the use of the Davis reagent or other similar oxaziridine reagents. This reaction mainly refers to the generation of α-hydroxy carbonyl compounds (acyloins) from ketones or esters. The reaction is carried out in a basic environment to generate the corresponding enolate from the ketone or ester. This reaction has been shown to work for amides.
In organic chemistry, the Lombardo methylenation is a name reaction that allows for the methylenation of carbonyl compounds with the use of Lombardo's reagent, which is a mix of zinc, dibromomethane, and titanium tetrachloride.

The epoxidation of allylic alcohols is a class of epoxidation reactions in organic chemistry. One implementation of this reaction is the Sharpless epoxidation. Early work showed that allylic alcohols give facial selectivity when using meta-chloroperoxybenzoic acid (m-CPBA) as an oxidant. This selectivity was reversed when the allylic alcohol was acetylated. This finding leads to the conclusion that hydrogen bonding played a key role in selectivity and the following model was proposed.













