A cement is a binder, a chemical substance used for construction that sets, hardens, and adheres to other materials to bind them together. Cement is seldom used on its own, but rather to bind sand and gravel (aggregate) together. Cement mixed with fine aggregate produces mortar for masonry, or with sand and gravel, produces concrete. Concrete is the most widely used material in existence and is behind only water as the planet's most-consumed resource.

A kiln is a thermally insulated chamber, a type of oven, that produces temperatures sufficient to complete some process, such as hardening, drying, or chemical changes. Kilns have been used for millennia to turn objects made from clay into pottery, tiles and bricks. Various industries use rotary kilns for pyroprocessing and to transform many other materials.

Liquid hydrogen (H2(l)) is the liquid state of the element hydrogen. Hydrogen is found naturally in the molecular H2 form.

Calcium oxide, commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term lime connotes calcium-containing inorganic compounds, in which carbonates, oxides, and hydroxides of calcium, silicon, magnesium, aluminium, and iron predominate. By contrast, quicklime specifically applies to the single compound calcium oxide. Calcium oxide that survives processing without reacting in building products, such as cement, is called free lime.

A combined cycle power plant is an assembly of heat engines that work in tandem from the same source of heat, converting it into mechanical energy. On land, when used to make electricity the most common type is called a combined cycle gas turbine (CCGT) plant, which is a kind of gas-fired power plant. The same principle is also used for marine propulsion, where it is called a combined gas and steam (COGAS) plant. Combining two or more thermodynamic cycles improves overall efficiency, which reduces fuel costs.
The heating value of a substance, usually a fuel or food, is the amount of heat released during the combustion of a specified amount of it.
Calcination is thermal treatment of a solid chemical compound (e.g. mixed carbonate ores) whereby the compound is raised to high temperature without melting under restricted supply of ambient oxygen (i.e. gaseous O2 fraction of air), generally for the purpose of removing impurities or volatile substances and/or to incur thermal decomposition.

Flue-gas desulfurization (FGD) is a set of technologies used to remove sulfur dioxide from exhaust flue gases of fossil-fuel power plants, and from the emissions of other sulfur oxide emitting processes such as waste incineration, petroleum refineries, cement and lime kilns.

A fossil fuel power station is a thermal power station which burns a fossil fuel, such as coal, oil, or natural gas, to produce electricity. Fossil fuel power stations have machinery to convert the heat energy of combustion into mechanical energy, which then operates an electrical generator. The prime mover may be a steam turbine, a gas turbine or, in small plants, a reciprocating gas engine. All plants use the energy extracted from the expansion of a hot gas, either steam or combustion gases. Although different energy conversion methods exist, all thermal power station conversion methods have their efficiency limited by the Carnot efficiency and therefore produce waste heat.

A thermal power station, also known as a thermal power plant, is a type of power station in which the heat energy generated from various fuel sources is converted to electrical energy. The heat from the source is converted into mechanical energy using a thermodynamic power cycle. The most common cycle involves a working fluid heated and boiled under high pressure in a pressure vessel to produce high-pressure steam. This high pressure-steam is then directed to a turbine, where it rotates the turbine's blades. The rotating turbine is mechanically connected to an electric generator which converts rotary motion into electricity. Fuels such as natural gas or oil can also be burnt directly in gas turbines, skipping the steam generation step. These plants can be of the open cycle or the more efficient combined cycle type.

Aberthaw Cement Works are cement works in the Vale of Glamorgan near the village of East Aberthaw in Wales.

Cement kilns are used for the pyroprocessing stage of manufacture of portland and other types of hydraulic cement, in which calcium carbonate reacts with silica-bearing minerals to form a mixture of calcium silicates. Over a billion tonnes of cement are made per year, and cement kilns are the heart of this production process: their capacity usually defines the capacity of the cement plant. As the main energy-consuming and greenhouse-gas–emitting stage of cement manufacture, improvement of kiln efficiency has been the central concern of cement manufacturing technology. Emissions from cement kilns are a major source of greenhouse gas emissions, accounting for around 2.5% of non-natural carbon emissions worldwide.
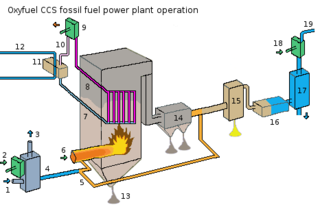
Oxy-fuel combustion is the process of burning a fuel using pure oxygen, or a mixture of oxygen and recirculated flue gas, instead of air. Since the nitrogen component of air is not heated, fuel consumption is reduced, and higher flame temperatures are possible. Historically, the primary use of oxy-fuel combustion has been in welding and cutting of metals, especially steel, since oxy-fuel allows for higher flame temperatures than can be achieved with an air-fuel flame. It has also received a lot of attention in recent decades as a potential carbon capture and storage technology.

A raw mill is the equipment used to grind raw materials into "rawmix" during the manufacture of cement. Rawmix is then fed to a cement kiln, which transforms it into clinker, which is then ground to make cement in the cement mill. The raw milling stage of the process effectively defines the chemistry of the finished cement, and has a large effect upon the efficiency of the whole manufacturing process.

A carbon dioxide scrubber is a piece of equipment that absorbs carbon dioxide (CO2). It is used to treat exhaust gases from industrial plants or from exhaled air in life support systems such as rebreathers or in spacecraft, submersible craft or airtight chambers. Carbon dioxide scrubbers are also used in controlled atmosphere (CA) storage and carbon capture and storage processes.

Annery kiln is a former limekiln of the estate of Annery, in the parish of Monkleigh, North Devon. It is situated on the left bank of the River Torridge near Half-Penny Bridge, built in 1835, which connects the parishes of Monkleigh and Weare Giffard. Running by it today is A386 road from Bideford to Great Torrington. Weare Giffard is the start of the tidal section of the River Torridge, and thus the kiln was sited here to import by river raw materials for the kiln, the product of which was lime fertiliser for use on inland agricultural fields. The old lime kiln is thus situated between the River Torridge and the now filled-in Rolle Canal built circa 1827 and railway that ran formerly from Bideford to Torrington, opened in 1872 and closed in 1966. The old trackbed now forms a stretch of the Tarka Trail.
The Nevada–Texas–Utah retort process was an above-ground shale oil extraction technology to produce shale oil, a type of synthetic crude oil. It heated oil shale in a sealed vessel (retort) causing its decomposition into shale oil, oil shale gas and spent residue. The process was developed in the 1920s and used for shale oil production in the United States and in Australia. The process was simple to operate; however, it was ceased from the operation because of a small capacity and labor extensiveness.

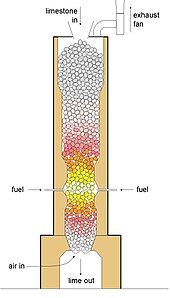
A Rumford furnace is a kiln for the industrial scale production in the 19th century of calcium oxide, popularly known as quicklime or burnt lime. It was named after its inventor, Benjamin Thompson, also known as Count Rumford, and is sometimes called a Rüdersdorf furnace after the location where it was first built and from where the design rapidly spread throughout Europe.
Calcium looping (CaL), or the regenerative calcium cycle (RCC), is a second-generation carbon capture technology. It is the most developed form of carbonate looping, where a metal (M) is reversibly reacted between its carbonate form (MCO3) and its oxide form (MO) to separate carbon dioxide from other gases coming from either power generation or an industrial plant. In the calcium looping process, the two species are calcium carbonate (CaCO3) and calcium oxide (CaO). The captured carbon dioxide can then be transported to a storage site, used in enhanced oil recovery or used as a chemical feedstock. Calcium oxide is often referred to as the sorbent.

A limepit is either a place where limestone is quarried, or a man-made pit used to burn lime stones in the same way that modern-day kilns and furnaces constructed of brick are now used above ground for the calcination of limestone (calcium carbonate, CaCO3) and by which quicklime (calcium oxide, CaO) is produced, an essential component in waterproofing and in wall plastering (plaster skim).