
Lipoxygenases are a family of (non-heme) iron-containing enzymes most of which catalyze the dioxygenation of polyunsaturated fatty acids in lipids containing a cis,cis-1,4- pentadiene into cell signaling agents that serve diverse roles as autocrine signals that regulate the function of their parent cells, paracrine signals that regulate the function of nearby cells, and endocrine signals that regulate the function of distant cells.
The crotonase family comprises mechanistically diverse proteins that share a conserved trimeric quaternary structure, the core of which consists of 4 turns of a (beta/beta/alpha)n superhelix.
In enzymology, a Delta12-fatty acid dehydrogenase (EC 1.14.99.33) is an enzyme that catalyzes the chemical reaction
In enzymology, a leucocyanidin oxygenase (EC 1.14.11.19) is an enzyme that catalyzes the chemical reaction
In enzymology, a 2,5-dihydroxypyridine 5,6-dioxygenase (EC 1.13.11.9) is an enzyme that catalyzes the chemical reaction
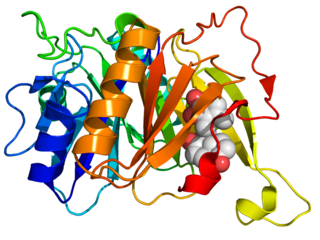
Biphenyl-2,3-diol 1,2-dioxygenase (EC 1.13.11.39) is an enzyme that catalyzes the chemical reaction
In enzymology, a linoleate 11-lipoxygenase (EC 1.13.11.45) is an enzyme that catalyzes the chemical reaction
In enzymology, a linoleate isomerase is an enzyme that catalyzes the chemical reaction

In enzymology, a prostaglandin-D synthase is an enzyme that catalyzes the chemical reaction
The enzyme hydroperoxide dehydratase (EC 4.2.1.92) catalyzes the chemical reaction
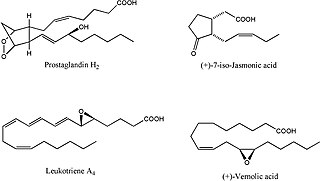
Oxylipins constitute a family of oxygenated natural products which are formed from fatty acids by pathways involving at least one step of dioxygen-dependent oxidation. Oxylipins are derived from polyunsaturated fatty acids (PUFAs) by COX enzymes (cyclooxygenases), by LOX enzymes (lipoxygenases), or by cytochrome P450 epoxygenase.
Linoleate 8R-lipoxygenase (EC 1.13.11.60, linoleic acid 8R-dioxygenase, 5,8-LDS (bifunctional enzyme), 7,8-LDS (bifunctional enzyme), 5,8-linoleate diol synthase (bifunctional enzyme), 7,8-linoleate diol synthase (bifunctional enzyme), PpoA) is an enzyme with systematic name linoleate:oxygen (8R)-oxidoreductase. This enzyme catalyses the following chemical reaction
5,8-linoleate diol synthase may refer to:
Linolenate 9R-lipoxygenase (EC 1.13.11.61, NspLOX, (9R)-LOX, linoleate 9R-dioxygenase) is an enzyme with systematic name alpha-linolenate:oxygen (9R)-oxidoreductase. This enzyme catalyses the following chemical reaction
Linoleate 10R-lipoxygenase (EC 1.13.11.62, 10R-DOX, (10R)-dioxygenase, 10R-dioxygenase) is an enzyme with systematic name linoleate:oxygen (10R)-oxidoreductase. This enzyme catalyses the following chemical reaction
9,12-octadecadienoate 8-hydroperoxide 8R-isomerase is an enzyme with systematic name (8R,9Z,12Z)-8-hydroperoxyoctadeca-9,12-dienoate hydroxymutase ( -5,8-dihydroxyoctadeca-9,12-dienoate-forming). This enzyme catalyses the following chemical reaction
9,12-octadecadienoate 8-hydroperoxide 8S-isomerase is an enzyme with systematic name (8R,9Z,12Z)-8-hydroperoxyoctadeca-9,12-dienoate hydroxymutase ( -7,8-dihydroxyoctadeca-9,12-dienoate-forming). This enzyme catalyses the following chemical reaction
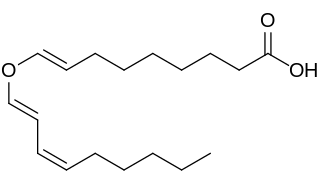
Divinylether fatty acids contain a fatty acid chemically combined with a doubly unsaturated carbon chain linked by an oxygen atom (ether). Fatty acid hydroperoxides generated by plant lipoxygenases from linoleic and linolenic acids are known to serve as substrates for a divinyl ether synthase which produces divinyl ether fatty acids. Up to date divinyl ethers were detected only within the plant kingdom.
Alpha-ketoglutarate-dependent hydroxylases are a major class of non-heme iron proteins that catalyse a wide range of reactions. These reactions include hydroxylation reactions, demethylations, ring expansions, ring closures, and desaturations. Functionally, the αKG-dependent hydroxylases are comparable to cytochrome P450 enzymes. Both use O2 and reducing equivalents as cosubstrates and both generate water.

Hydroperoxide lyases are enzymes that catalyze the cleavage of C-C bonds in the hydroperoxides of fatty acids. They belong to the cytochrome P450 enzyme family.






