
B cells, also known as B lymphocytes, are a type of white blood cell of the lymphocyte subtype. They function in the humoral immunity component of the adaptive immune system. B cells produce antibody molecules which may be either secreted or inserted into the plasma membrane where they serve as a part of B-cell receptors. When a naïve or memory B cell is activated by an antigen, it proliferates and differentiates into an antibody-secreting effector cell, known as a plasmablast or plasma cell. Additionally, B cells present antigens and secrete cytokines. In mammals, B cells mature in the bone marrow, which is at the core of most bones. In birds, B cells mature in the bursa of Fabricius, a lymphoid organ where they were first discovered by Chang and Glick, which is why the 'B' stands for bursa and not bone marrow as commonly believed.

Natural killer cells, also known as NK cells or large granular lymphocytes (LGL), are a type of cytotoxic lymphocyte critical to the innate immune system that belong to the rapidly expanding family of known innate lymphoid cells (ILC) and represent 5–20% of all circulating lymphocytes in humans. The role of NK cells is analogous to that of cytotoxic T cells in the vertebrate adaptive immune response. NK cells provide rapid responses to virus-infected cell and other intracellular pathogens acting at around 3 days after infection, and respond to tumor formation. Typically, immune cells detect the antigen presented on major histocompatibility complex (MHC) on infected cell surfaces, triggering cytokine release, causing the death of the infected cell by lysis or apoptosis. NK cells are unique, however, as they have the ability to recognize and kill stressed cells in the absence of antibodies and MHC, allowing for a much faster immune reaction. They were named "natural killers" because of the notion that they do not require activation to kill cells that are missing "self" markers of MHC class I. This role is especially important because harmful cells that are missing MHC I markers cannot be detected and destroyed by other immune cells, such as T lymphocyte cells.
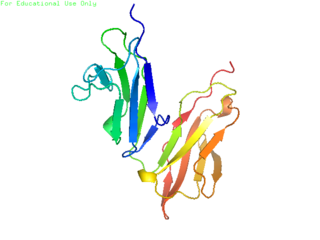
CD32, also known as FcγRII or FCGR2, is a surface receptor glycoprotein belonging to the Ig gene superfamily. CD32 can be found on the surface of a variety of immune cells. CD32 has a low-affinity for the Fc region of IgG antibodies in monomeric form, but high affinity for IgG immune complexes. CD32 has two major functions: cellular response regulation, and the uptake of immune complexes. Cellular responses regulated by CD32 include phagocytosis, cytokine stimulation, and endocytic transport. Dysregulated CD32 is associated with different forms of autoimmunity, including systemic lupus erythematosus. In humans, there are three major CD32 subtypes: CD32A, CD32B, and CD32C. While CD32A and CD32C are involved in activating cellular responses, CD32B is inhibitory.

Plasma cells, also called plasma B cells or effector B cells, are white blood cells that originate in the lymphoid organs as B lymphocytes and secrete large quantities of proteins called antibodies in response to being presented specific substances called antigens. These antibodies are transported from the plasma cells by the blood plasma and the lymphatic system to the site of the target antigen, where they initiate its neutralization or destruction. B cells differentiate into plasma cells that produce antibody molecules closely modeled after the receptors of the precursor B cell.

In immunology, a memory B cell (MBC) is a type of B lymphocyte that forms part of the adaptive immune system. These cells develop within germinal centers of the secondary lymphoid organs. Memory B cells circulate in the blood stream in a quiescent state, sometimes for decades. Their function is to memorize the characteristics of the antigen that activated their parent B cell during initial infection such that if the memory B cell later encounters the same antigen, it triggers an accelerated and robust secondary immune response. Memory B cells have B cell receptors (BCRs) on their cell membrane, identical to the one on their parent cell, that allow them to recognize antigen and mount a specific antibody response.

CD23, also known as Fc epsilon RII, or FcεRII, is the "low-affinity" receptor for IgE, an antibody isotype involved in allergy and resistance to parasites, and is important in regulation of IgE levels. Unlike many of the antibody receptors, CD23 is a C-type lectin. It is found on mature B cells, activated macrophages, eosinophils, follicular dendritic cells, and platelets.
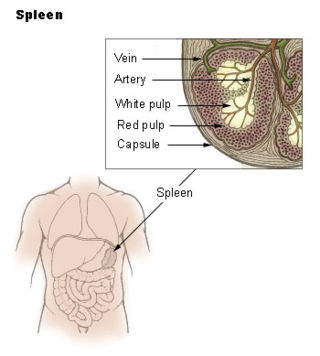
The marginal zone is the region at the interface between the non-lymphoid red pulp and the lymphoid white-pulp of the spleen.

In immunology, a Fc receptor is a protein found on the surface of certain cells – including, among others, B lymphocytes, follicular dendritic cells, natural killer cells, macrophages, neutrophils, eosinophils, basophils, human platelets, and mast cells – that contribute to the protective functions of the immune system. Its name is derived from its binding specificity for a part of an antibody known as the Fc region. Fc receptors bind to antibodies that are attached to infected cells or invading pathogens. Their activity stimulates phagocytic or cytotoxic cells to destroy microbes, or infected cells by antibody-mediated phagocytosis or antibody-dependent cell-mediated cytotoxicity. Some viruses such as flaviviruses use Fc receptors to help them infect cells, by a mechanism known as antibody-dependent enhancement of infection.

Germinal centers or germinal centres (GCs) are transiently formed structures within B cell zone (follicles) in secondary lymphoid organs – lymph nodes, ileal Peyer's patches, and the spleen – where mature B cells are activated, proliferate, differentiate, and mutate their antibody genes during a normal immune response; most of the germinal center B cells (BGC) are removed by tingible body macrophages. There are several key differences between naive B cells and GC B cells, including level of proliferative activity, size, metabolic activity and energy production. The B cells develop dynamically after the activation of follicular B cells by T-dependent antigen. The initiation of germinal center formation involves the interaction between B and T cells in the interfollicular area of the lymph node, CD40-CD40L ligation, NF-kB signaling and expression of IRF4 and BCL6.

An immune complex, sometimes called an antigen-antibody complex or antigen-bound antibody, is a molecule formed from the binding of multiple antigens to antibodies. The bound antigen and antibody act as a unitary object, effectively an antigen of its own with a specific epitope. After an antigen-antibody reaction, the immune complexes can be subject to any of a number of responses, including complement deposition, opsonization, phagocytosis, or processing by proteases. Red blood cells carrying CR1-receptors on their surface may bind C3b-coated immune complexes and transport them to phagocytes, mostly in liver and spleen, and return to the general circulation.
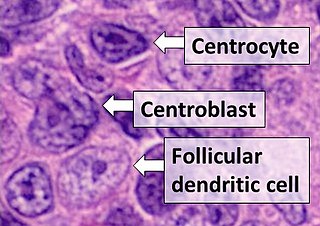
Follicular dendritic cells (FDC) are cells of the immune system found in primary and secondary lymph follicles of the B cell areas of the lymphoid tissue. Unlike dendritic cells (DC), FDCs are not derived from the bone-marrow hematopoietic stem cell, but are of mesenchymal origin. Possible functions of FDC include: organizing lymphoid tissue's cells and microarchitecture, capturing antigen to support B cell, promoting debris removal from germinal centers, and protecting against autoimmunity. Disease processes that FDC may contribute include primary FDC-tumor, chronic inflammatory conditions, HIV-1 infection development, and neuroinvasive scrapie.

B-lymphocyte antigen CD19, also known as CD19 molecule, B-Lymphocyte Surface Antigen B4, T-Cell Surface Antigen Leu-12 and CVID3 is a transmembrane protein that in humans is encoded by the gene CD19. In humans, CD19 is expressed in all B lineage cells. Contrary to some early doubts, human plasma cells do express CD19, as confirmed by others. CD19 plays two major roles in human B cells: on the one hand, it acts as an adaptor protein to recruit cytoplasmic signaling proteins to the membrane; on the other, it works within the CD19/CD21 complex to decrease the threshold for B cell receptor signaling pathways. Due to its presence on all B cells, it is a biomarker for B lymphocyte development, lymphoma diagnosis and can be utilized as a target for leukemia immunotherapies.
CD70 is a protein that in humans is encoded by CD70 gene. CD70 is also known as a ligand for CD27.
B1 cells are a sub-class of B cell lymphocytes that are involved in the humoral immune response. They are not part of the adaptive immune system, as they have no memory, but otherwise, B1 cells perform many of the same roles as other B cells: making antibodies against antigens and acting as antigen-presenting cells. These B1 cells are commonly found in peripheral sites, but less commonly found in the blood. These cells are involved in antibody response during an infection or vaccination.

G-protein coupled receptor 183 also known as Epstein-Barr virus-induced G-protein coupled receptor 2 (EBI2) is a protein (GPCR) expressed on the surface of some immune cells, namely B cells and T cells; in humans it is encoded by the GPR183 gene. Expression of EBI2 is one critical mediator of immune cell localization within lymph nodes, responsible in part for the coordination of B cell, T cell, and dendritic cell movement and interaction following antigen exposure. EBI2 is a receptor for oxysterols. The most potent activator is 7α,25-dihydroxycholesterol (7α,25-OHC), with other oxysterols exhibiting varying affinities for the receptor. Oxysterol gradients drive chemotaxis, attracting the EBI2-expressing cells to locations of high ligand concentration. The GPR183 gene was identified due to its upregulation during Epstein-Barr virus infection of the Burkitt's lymphoma cell line BL41, hence its name: EBI2.
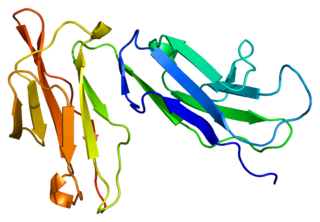
Fc fragment of IgG receptor IIb is a low affinity inhibitory receptor for the Fc region of immunoglobulin gamma (IgG). FCGR2B participates in the phagocytosis of immune complexes and in the regulation of antibody production by B lymphocytes.
Within the immune system, Follicular B cells are a type of B cell that reside in primary and secondary lymphoid follicles of secondary and tertiary lymphoid organs, including spleen and lymph nodes. Antibody responses against proteins are believed to involve follicular B cell pathways in secondary lymphoid organs.

Follicular helper T cells (also known as follicular B helper T cells and abbreviated as TFH), are antigen-experienced CD4+ T cells found in the periphery within B cell follicles of secondary lymphoid organs such as lymph nodes, spleen and Peyer's patches, and are identified by their constitutive expression of the B cell follicle homing receptor CXCR5. Upon cellular interaction and cross-signaling with their cognate follicular (Fo B) B cells, TFH cells trigger the formation and maintenance of germinal centers through the expression of CD40 ligand (CD40L) and the secretion of IL-21 and IL-4. TFH cells also migrate from T cell zones into these seeded germinal centers, predominantly composed of rapidly dividing B cells mutating their Ig genes. Within germinal centers, TFH cells play a critical role in mediating the selection and survival of B cells that go on to differentiate either into long-lived plasma cells capable of producing high affinity antibodies against foreign antigen, or germinal center-dependent memory B cells capable of quick immune re-activation in the future if ever the same antigen is re-encountered. TFH cells are also thought to facilitate negative selection of potentially autoimmune-causing mutated B cells in the germinal center. However, the biomechanisms by which TFH cells mediate germinal center tolerance are yet to be fully understood.

A centroblast generally refers to an activated B cell that is enlarged and is rapidly proliferating in the germinal center of a lymphoid follicle. They are specifically located in the dark zone of the germinal center. Centroblasts form from naive B cells being exposed to follicular dendritic cell cytokines, such as IL-6, IL-15, 8D6, and BAFF. Stimulation from helper T cells is also required for centroblast development. Interaction between CD40 ligand on an activated T helper cell and the B cell CD40 receptor induces centroblasts to express activation-induced cytidine deaminase, leading to somatic hypermutation, allowing the B cell receptor to potentially gain stronger affinity for an antigen. In the absence of FDC and helper T cell stimulation, centroblasts are unable to differentiate and will undergo CD95-mediated apoptosis.
B10 cells are a sub-class of regulatory B-cells that are involved in inhibiting immune responses in both humans and mice. B10 cells are named for their ability to produce inhibitory interleukin: Interleukin-10 (IL-10). One of their unique abilities is that they suppress the innate and adaptive immune signals, making them important for regulating the inflammatory response. Like the B cell, the B10 cell requires antigen specific binding to the surface of CD5 receptor to illicit a response from the T-cell. Once an antigen binds to the CD19 receptor, immediate downregulation in B-cell receptor (BCR) signal expression occurs and mediates the release of IL-10 cytokines. In mice and humans, B10 cells are distinguishable in their expression of measurable IL-10 due to the lack of unique cell surface markers expressed by regulatory B cells. However, IL-10 competence is not limited to any one subset of B cells. B10 cells do not possess unique phenotypic markers or transcription factors for further identification. B10 cells predominantly localize in the spleen, though they are also found in the blood, lymph nodes, Peyer's patches, intestinal tissues, central nervous system, and peritoneal cavity. B10 cells proliferate during inflammatory and disease responses.
















