
Illicium verum is a medium-sized evergreen tree native to northeast Vietnam and southwest China. A spice commonly called star anise, staranise, star anise seed, star aniseed, star of anise, Chinese star anise, or badian that closely resembles anise in flavor is obtained from the star-shaped pericarps of the fruit of I. verum which are harvested just before ripening. Star anise oil is a highly fragrant oil used in cooking, perfumery, soaps, toothpastes, mouthwashes, and skin creams. Until 2012, when they switched to using a bacterial source, Roche Pharmaceuticals used up to 90% of the world's annual star anise crop to produce shikimic acid, a chemical intermediate used in the synthesis of oseltamivir (Tamiflu).
Shikimic acid, more commonly known as its anionic form shikimate, is a cyclohexene, a cyclitol and a cyclohexanecarboxylic acid. It is an important biochemical metabolite in plants and microorganisms. Its name comes from the Japanese flower shikimi, from which it was first isolated in 1885 by Johan Fredrik Eykman. The elucidation of its structure was made nearly 50 years later.

Prephenic acid, commonly also known by its anionic form prephenate, is an intermediate in the biosynthesis of the aromatic amino acids phenylalanine and tyrosine, as well as of a large number of secondary metabolites of the shikimate pathway.

Illicium anisatum, with common names Japanese star anise, Aniseed tree, and sacred Anise tree, known in Japan as Shikimi, is a tree closely related to the Chinese star anise. Since it is highly toxic, the fruit is not edible; instead, the dried and powdered leaves are burned as incense in Japan. Its branches and evergreen leaves are considered highly sacred by Japanese Buddhists due to aversion from insects and their ability to remain fresh after pruning.
Longifolene is the common chemical name of a naturally occurring, oily liquid hydrocarbon found primarily in the high-boiling fraction of certain pine resins. The name is derived from that of a pine species from which the compound was isolated, Chemically, longifolene is a tricyclic sesquiterpene. This molecule is chiral, and the enantiomer commonly found in pines and other higher plants exhibits a positive optical rotation of +42.73°. The other enantiomer is found in small amounts in certain fungi and liverworts.

Paclitaxel total synthesis in organic chemistry is a major ongoing research effort in the total synthesis of paclitaxel (Taxol). This diterpenoid is an important drug in the treatment of cancer but, also expensive because the compound is harvested from a scarce resource, namely the Pacific yew. Not only is the synthetic reproduction of the compound itself of great commercial and scientific importance, but it also opens the way to paclitaxel derivatives not found in nature but with greater potential.

Danishefsky's diene is an organosilicon compound and a diene with the formal name trans-1-methoxy-3-trimethylsilyloxy-buta-1,3-diene named after Samuel J. Danishefsky. Because the diene is very electron-rich it is a very reactive reagent in Diels-Alder reactions. This diene reacts rapidly with electrophilic alkenes, such as maleic anhydride. The methoxy group promotes highly regioselective additions. The diene is known to react with amines, aldehydes, alkenes and alkynes. Reactions with imines and nitro-olefins have been reported.

Isopentenyl pyrophosphate isomerase, also known as Isopentenyl-diphosphate delta isomerase, is an isomerase that catalyzes the conversion of the relatively un-reactive isopentenyl pyrophosphate (IPP) to the more-reactive electrophile dimethylallyl pyrophosphate (DMAPP). This isomerization is a key step in the biosynthesis of isoprenoids through the mevalonate pathway and the MEP pathway.
Iodolactonization is an organic reaction that forms a ring by the addition of an oxygen and iodine across a carbon-carbon double bond. It is an intramolecular variant of the halohydrin synthesis reaction. The reaction was first reported by M. J. Bougalt in 1904 and has since become one of the most effective ways to synthesize lactones. Strengths of the reaction include the mild conditions and incorporation of the versatile iodine atom into the product.
Robert S. Coleman is an American chemistry professor and researcher. Coleman was a faculty member at both Ohio State University and the University of South Carolina. At Ohio State, he was on the faculty in the Department of Chemistry from 1996 to 2012, having moved to Ohio State as an associate professor from the University of South Carolina. At USC, Coleman taught as assistant professor from 1989 to 1995, and then as associate professor from 1995 to 1996. In 1996, he accepted a faculty position at Ohio State University to teach Organic Chemistry, where he was an associate professor from 1996 until 2000. He was promoted to full professor in 2000, teaching Organic Chemistry up until his retirement in 2012. He received his Ph.D. degree working with Professor Dale L. Boger, completing the first total synthesis of the antitumor agent CC-1065. He was subsequently an NIH postdoctoral fellow at Yale University with Professor Samuel J. Danishefsky, where he completed, the first total synthesis of the aglycone of the antitumor agent calicheamicin.
The enzyme aristolochene synthase catalyzes the chemical reaction
The enzyme germacrene-A synthase catalyzes the chemical reaction
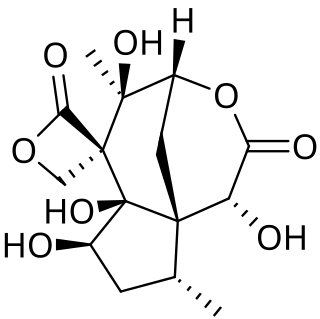
Anisatin is an extremely toxic, insecticidally active component of the Shikimi plant. The lethal dose is 1 mg/kg (i.p.) in mice. Symptoms begin to appear about 1–6 hours after ingestion, beginning with gastrointestinal ailments, such as diarrhea, vomiting, and stomach pain, followed by nervous system excitation, seizures, loss of consciousness, and respiratory paralysis, which is the ultimate cause of death.
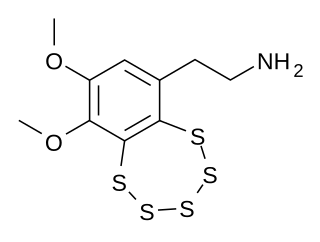
Varacin is a bicyclic organosulfur compound originally found in marine Ascidiacea from the Polycitor genus. It contains an unusual pentathiepin ring which reacts with DNA, and varacin and synthetic analogues have been investigated for their antimicrobial and antitumour properties. Because of its potent biological activity and unusual and challenging ring system, it has been a popular target of efforts toward its total synthesis.

Absinthin is a naturally produced triterpene lactone from the plant Artemisia absinthium (Wormwood). It constitutes one of the most bitter chemical agents responsible for absinthe's distinct taste. The compound shows biological activity and has shown promise as an anti-inflammatory agent, and should not to be confused with thujone, a neurotoxin also found in Artemisia absinthium.

Juvabione, historically known as the paper factor, is the methyl ester of todomatuic acid, both of which are sesquiterpenes (C15) found in the wood of true firs of the genus Abies. They occur naturally as part of a mixture of sesquiterpenes based upon the bisabolane scaffold. Sesquiterpenes of this family are known as insect juvenile hormone analogues (IJHA) because of their ability to mimic juvenile activity in order to stifle insect reproduction and growth. These compounds play important roles in conifers as the second line of defense against insect induced trauma and fungal pathogens.
The Saegusa–Ito oxidation is a chemical reaction used in organic chemistry. It was discovered in 1978 by Takeo Saegusa and Yoshihiko Ito as a method to introduce α-β unsaturation in carbonyl compounds. The reaction as originally reported involved formation of a silyl enol ether followed by treatment with palladium(II) acetate and benzoquinone to yield the corresponding enone. The original publication noted its utility for regeneration of unsaturation following 1,4-addition with nucleophiles such as organocuprates.
5-Epiaristolochene synthase is an enzyme with systematic name (2E,6E)-farnesyl-diphosphate diphosphate-lyase ( -5-epiaristolochene-forming). This enzyme catalyses the following chemical reaction

Jiadifenolide is a sesquiterpenoid natural product with neurotrophic activity, found in Illicium jiadifengpi. Its biological activity and congested polycyclic structure have made it a popular target for total synthesis.

Arglabin is a sesquiterpene lactone belonging to the guaianolide subclass bearing a 5,7,5-tricyclic ring system which is known to inhibit farnesyl transferase. It is characterized by an epoxide on the cycloheptane as well as an exocyclic methylene group that is conjugated with the carbonyl of the lactone. Arglabin is extracted from Artemisia glabella, a species of wormwood, found in the Karaganda Region of Kazakhstan. Arglabin and its derivatives are biologically active and demonstrate promising antitumor activity and cytoxocity against varying tumor cell lines.












