Related Research Articles
Methanogens are microorganisms that produce methane as a metabolic byproduct in hypoxic conditions. They belong to the domain Archaea and are members of the phylum Euryarchaeota. Methanogens are common in wetlands, where they are responsible for marsh gas, and can occur in the digestive tracts of animals including ruminants and humans, where they are responsible for the methane content of belching and flatulence. In marine sediments, the biological production of methane, termed methanogenesis, is generally confined to where sulfates are depleted below the top layers and methanogens play an indispensable role in anaerobic wastewater treatments. Other methanogens are extremophiles, found in environments such as hot springs and submarine hydrothermal vents as well as in the "solid" rock of Earth's crust, kilometers below the surface.

Oxaloacetate decarboxylase is a carboxy-lyase involved in the conversion of oxaloacetate into pyruvate.
Clostripain is a proteinase that cleaves proteins on the carboxyl peptide bond of arginine. It was isolated from Clostridium histolyticum. The isoelectric point of the enzyme is 4.8-4.9, and optimum pH is 7.4~7.8. The composition of the enzyme is indicated to be of two chains of relative molecular mass 45,000 and 12,500.

Glycerol-3-phosphate dehydrogenase (GPDH) is an enzyme that catalyzes the reversible redox conversion of dihydroxyacetone phosphate to sn-glycerol 3-phosphate.
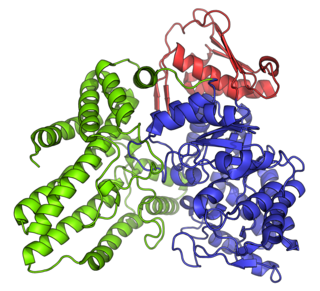
The enzyme α-N-acetylglucosaminidase is a protein associated with Sanfilippo syndrome, with systematic name α-N-acetyl-D-glucosaminide N-acetylglucosaminohydrolase. It catalyses the hydrolysis of terminal non-reducing N-acetyl-D-glucosamine residues in N-acetyl-α-D-glucosaminides, and also UDP-N-acetylglucosamine.
In enzymology, a mycothiol-dependent formaldehyde dehydrogenase (EC 1.1.1.306) is an enzyme that catalyzes the chemical reaction

Alcohol dehydrogenase 1C is an enzyme that in humans is encoded by the ADH1C gene.

17β-Hydroxysteroid dehydrogenase 1 (17β-HSD1) is an enzyme that in humans is encoded by the HSD17B1 gene. This enzyme oxidizes or reduces the C17 hydroxy/keto group of androgens and estrogens and hence is able to regulate the potency of these sex steroids

The short-chain dehydrogenases/reductases family (SDR) is a very large family of enzymes, most of which are known to be NAD- or NADP-dependent oxidoreductases. As the first member of this family to be characterised was Drosophila alcohol dehydrogenase, this family used to be called 'insect-type', or 'short-chain' alcohol dehydrogenases. Most members of this family are proteins of about 250 to 300 amino acid residues. Most dehydrogenases possess at least 2 domains, the first binding the coenzyme, often NAD, and the second binding the substrate. This latter domain determines the substrate specificity and contains amino acids involved in catalysis. Little sequence similarity has been found in the coenzyme binding domain although there is a large degree of structural similarity, and it has therefore been suggested that the structure of dehydrogenases has arisen through gene fusion of a common ancestral coenzyme nucleotide sequence with various substrate specific domains.

Alcohol dehydrogenase class 4 mu/sigma chain is an enzyme that in humans is encoded by the ADH7 gene.

4-trimethylaminobutyraldehyde dehydrogenase is an enzyme that in humans is encoded by the ALDH9A1 gene.

Morpheeins are proteins that can form two or more different homo-oligomers, but must come apart and change shape to convert between forms. The alternate shape may reassemble to a different oligomer. The shape of the subunit dictates which oligomer is formed. Each oligomer has a finite number of subunits (stoichiometry). Morpheeins can interconvert between forms under physiological conditions and can exist as an equilibrium of different oligomers. These oligomers are physiologically relevant and are not misfolded protein; this distinguishes morpheeins from prions and amyloid. The different oligomers have distinct functionality. Interconversion of morpheein forms can be a structural basis for allosteric regulation, an idea noted many years ago, and later revived. A mutation that shifts the normal equilibrium of morpheein forms can serve as the basis for a conformational disease. Features of morpheeins can be exploited for drug discovery. The dice image represents a morpheein equilibrium containing two different monomeric shapes that dictate assembly to a tetramer or a pentamer. The one protein that is established to function as a morpheein is porphobilinogen synthase, though there are suggestions throughout the literature that other proteins may function as morpheeins.
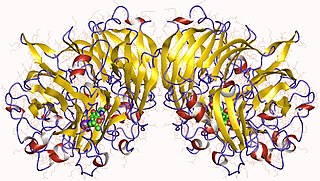
Methanol dehydrogenase (cytochrome c) (EC 1.1.2.7, methanol dehydrogenase, MDH) is an enzyme with systematic name methanol:cytochrome c oxidoreductase. This enzyme catalyses the following chemical reaction
Nitrate reductase (quinone) (EC 1.7.5.1, nitrate reductase A, nitrate reductase Z, quinol/nitrate oxidoreductase, quinol-nitrate oxidoreductase, quinol:nitrate oxidoreductase, NarA, NarZ, NarGHI) is an enzyme with systematic name nitrite:quinone oxidoreductase. This enzyme catalyses the following chemical reaction
(Methyl-Co methanol-specific corrinoid protein):coenzyme M methyltransferase is an enzyme with systematic name methylated methanol-specific corrinoid protein:coenzyme M methyltransferase. This enzyme catalyses the following chemical reaction
Hypodermin C is an enzyme. This enzyme catalyses the following chemical reaction
Serralysin is an enzyme. This enzyme catalyses the following chemical reaction
Atrolysin C is an enzyme. This enzyme catalyses the following chemical reaction
The H+-translocating F420H2 Dehydrogenase (F420H2DH) Family(TC# 3.D.9) is a member of the Na+ transporting Mrp superfamily. A single F420H2 dehydrogenase (also referred to as F420H2:quinol oxidoreductase) from the methanogenic archaeon, Methanosarcina mazei Gö1, has been shown to be a redox driven proton pump. The F420H2DH of M. mazei has a molecular size of about 120 kDa and contains Fe-S clusters and FAD. A similar five-subunit enzyme has been isolated from Methanolobus tindarius. The sulfate-reducing Archaeoglobus fulgidus (and several other archaea) also have this enzyme.
In enzymology, a formylmethanofuran dehydrogenase (EC 1.2.99.5) is an enzyme that catalyzes the chemical reaction:
References
- ↑ König, Helmut, and Karl O. Stetter. "Isolation and characterization of Methanolobus tindarius, sp. nov., a coccoid methanogen growing only on methanol and methylamines." Zentralblatt für Bakteriologie Mikrobiologie und Hygiene: I. Abt. Originale C: Allgemeine, angewandte und ökologische Mikrobiologie 3.4 (1982): 478-490.
- ↑ Sue-Yao Wu; Mei-Chin Lai (2010-12-10). "Methanogenic Archaea Isolated from Taiwan's Chelungpu Fault". Appl. Environ. Microbiol. 27 (3): 830–838. doi:10.1128/AEM.01539-10. PMC 3028716 . PMID 21148697.