Basil (, ; Ocimum basilicum, also called great basil, is a culinary herb of the family Lamiaceae. It is a tender plant, and is used in cuisines worldwide. In Western cuisine, the generic term "basil" refers to the variety also known as sweet basil or Genovese basil. Basil is native to tropical regions from Central Africa to Southeast Asia. In temperate climates basil is treated as an annual plant, however, basil can be grown as a short-lived perennial or biennial in warmer horticultural zones with tropical or Mediterranean climates.

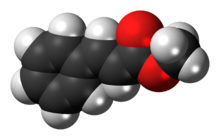
Cinnamaldehyde is an organic compound with the formula() C6H5CH=CHCHO. Occurring naturally as predominantly the trans (E) isomer, it gives cinnamon its flavor and odor. It is a phenylpropanoid that is naturally synthesized by the shikimate pathway. This pale yellow, viscous liquid occurs in the bark of cinnamon trees and other species of the genus Cinnamomum. The essential oil of cinnamon bark is about 90% cinnamaldehyde. Cinnamaldehyde decomposes to styrene because of oxidation as a result of bad storage or transport conditions. Styrene especially forms in high humidity and high temperatures. This is the reason why cinnamon contains small amounts of styrene.

Cinnamic acid is an organic compound with the formula C6H5-CH=CH-COOH. It is a white crystalline compound that is slightly soluble in water, and freely soluble in many organic solvents. Classified as an unsaturated carboxylic acid, it occurs naturally in a number of plants. It exists as both a cis and a trans isomer, although the latter is more common.

Eugenol is an allyl chain-substituted guaiacol, a member of the allylbenzene class of chemical compounds. It is a colorless to pale yellow, aromatic oily liquid extracted from certain essential oils especially from clove, nutmeg, cinnamon, basil and bay leaf. It is present in concentrations of 80–90% in clove bud oil and at 82–88% in clove leaf oil. Eugenol has a pleasant, spicy, clove-like scent. The name is derived from Eugenia caryophyllata, the former Linnean nomenclature term for cloves. The currently accepted name is Syzygium aromaticum.

Linalool refers to two enantiomers of a naturally occurring terpene alcohol found in many flowers and spice plants. Linalool has multiple commercial applications, the majority of which are based on its pleasant scent. A colorless oil, linalool is classified as an acyclic monoterpenoid. In plants, it is a metabolite, a volatile oil component, an antimicrobial agent, and an aroma compound. Linalool has uses in manufacturing of soaps, fragrances, food additives as flavors, household products, and insecticides. Esters of linalool are referred to as linalyl, e.g. linalyl pyrophosphate, an isomer of geranyl pyrophosphate.

The garden strawberry is a widely grown hybrid species of the genus Fragaria, collectively known as the strawberries, which are cultivated worldwide for their fruit. The fruit is widely appreciated for its characteristic aroma, bright red color, juicy texture, and sweetness. It is consumed in large quantities, either fresh or in such prepared foods as jam, juice, pies, ice cream, milkshakes, and chocolates. Artificial strawberry flavorings and aromas are also widely used in products such as candy, soap, lip gloss, perfume, and many others.

Carrion flowers, also known as corpse flowers or stinking flowers, are mimetic flowers that emit an odor that smells like rotting flesh. Apart from the scent, carrion flowers often display additional characteristics that contribute to the mimesis of a decaying corpse. These include their specific coloration, the presence of setae and orifice-like flower architecture. Carrion flowers attract mostly scavenging flies and beetles as pollinators. Some species may trap the insects temporarily to ensure the gathering and transfer of pollen.

Estragole is a phenylpropene, a natural organic compound. Its chemical structure consists of a benzene ring substituted with a methoxy group and an allyl group. It is an isomer of anethole, differing with respect to the location of the double bond. It is a colorless liquid, although impure samples can appear yellow. It is a component of various trees and plants, including turpentine, anise, fennel, bay, tarragon, and basil. It is used in the preparation of fragrances.

Chavicol (p-allylphenol) is a natural phenylpropene, a type of organic compound. Its chemical structure consists of a benzene ring substituted with a hydroxy group and a propenyl group. It is a colorless liquid found together with terpenes in betel oil.

Eucalyptus oil is the generic name for distilled oil from the leaf of Eucalyptus, a genus of the plant family Myrtaceae native to Australia and cultivated worldwide. Eucalyptus oil has a history of wide application, as a pharmaceutical, antiseptic, repellent, flavouring, fragrance and industrial uses. The leaves of selected Eucalyptus species are steam distilled to extract eucalyptus oil.

Cinnamon basil is a type of basil. The term "cinnamon basil" can refer to a number of different varieties of basil, including as a synonym for Thai basil, as a particular cultivar of Thai basil, and as a separate cultivar in its own right. This article discusses the latter type.

Ocimum americanum, known as American basil, lime basil, or hoary basil, is a species of annual herb in the family Lamiaceae. Despite the misleading name, it is native to Africa, the Indian Subcontinent, China, and Southeast Asia. The species is naturalized in Queensland, Christmas Island, and parts of tropical America.

Ocotea quixos is a species of evergreen tree in the family Lauraceae, native to Ecuador and Colombia. It is one of the South American trees with a cinnamon-like aroma and is used as a spice called ispinku in Southern Quechua or ishpinku in Kichwa.

Ocimum campechianum is a plant species in the family Lamiaceae, widespread across Mexico, Central America, South America, the West Indies, and Florida.

Greek basil is a flowering herb and cultivar of basil.