Toluene, also known as toluol, is an aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH₃) attached to a phenyl group. As such, its systematic IUPAC name is methylbenzene. Toluene is predominantly used as an industrial feedstock and a solvent.
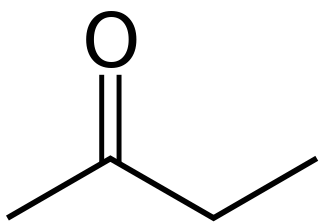
Butanone, also known as methyl ethyl ketone (MEK), is an organic compound with the formula CH3C(O)CH2CH3. This colourless liquid ketone has a sharp, sweet odor reminiscent of acetone. It is produced industrially on a large scale, but occurs in nature only in trace amounts. It is partially soluble in water, and is commonly used as an industrial solvent. It is an isomer of another solvent, tetrahydrofuran.
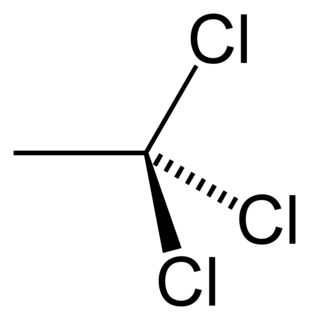
The organic compound 1,1,1-trichloroethane, also known as methyl chloroform, is a chloroalkane. This colorless, sweet-smelling liquid was once produced industrially in large quantities for use as a solvent. It is regulated by the Montreal Protocol as an ozone-depleting substance and its use is being rapidly phased out.
Acetamide (systematic name: ethanamide) is an organic compound with the formula CH3CONH2. It is the simplest amide derived from acetic acid. It finds some use as a plasticizer and as an industrial solvent. The related compound N,N-dimethylacetamide (DMA) is more widely used, but it is not prepared from acetamide. Acetamide can be considered an intermediate between acetone, which has two methyl (CH3) groups either side of the carbonyl (CO), and urea which has two amide (NH2) groups in those locations.
Methylamine is an organic compound with a formula of CH3NH2. This colorless gas is a derivative of ammonia, but with one hydrogen atom being replaced by a methyl group. It is the simplest primary amine.

Methyl acetate, also known as MeOAc, acetic acid methyl ester or methyl ethanoate, is a carboxylate ester with the formula CH3COOCH3. It is a flammable liquid with a characteristically pleasant smell reminiscent of some glues and nail polish removers. Methyl acetate is occasionally used as a solvent, being weakly polar and lipophilic, but its close relative ethyl acetate is a more common solvent being less toxic and less soluble in water. Methyl acetate has a solubility of 25% in water at room temperature. At elevated temperature its solubility in water is much higher. Methyl acetate is not stable in the presence of strong aqueous bases or aqueous acids. Methyl acetate is not considered a VOC in the USA.

2-Butanol, or sec-butanol, is an organic compound with formula CH3CH(OH)CH2CH3. This secondary alcohol is a flammable, colorless liquid that is soluble in three parts water and completely miscible with organic solvents. It is produced on a large scale, primarily as a precursor to the industrial solvent methyl ethyl ketone. 2-Butanol is chiral and thus can be obtained as either of two stereoisomers designated as (R)-(−)-2-butanol and (S)-(+)-2-butanol. It is normally encountered as a 1:1 mixture of the two stereoisomers — a racemic mixture.

Acetone, or propanone, is an organic compound with the formula (CH3)2CO. It is the simplest and smallest ketone. It is a colourless, highly volatile and flammable liquid with a characteristic pungent odour.
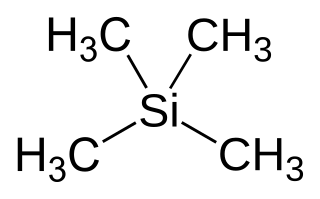
Tetramethylsilane (abbreviated as TMS) is the organosilicon compound with the formula Si(CH3)4. It is the simplest tetraorganosilane. Like all silanes, the TMS framework is tetrahedral. TMS is a building block in organometallic chemistry but also finds use in diverse niche applications.

Methyl isobutyl ketone (MIBK) is the organic compound with the formula (CH3)2CHCH2C(O)CH3. This colourless liquid, a ketone, is used as a solvent for gums, resins, paints, varnishes, lacquers, and nitrocellulose.
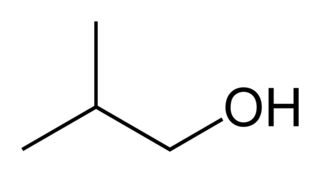
Isobutanol (IUPAC nomenclature: 2-methylpropan-1-ol) is an organic compound with the formula (CH3)2CHCH2OH (sometimes represented as i-BuOH). This colorless, flammable liquid with a characteristic smell is mainly used as a solvent either directly or as its esters. Its isomers, the other butanols, include n-butanol, 2-butanol, and tert-butanol, all of which are important industrially.
Glycol ethers are a group of solvents based on alkyl ethers of ethylene glycol or propylene glycol commonly used in paints and cleaners. These solvents typically have a higher boiling point, together with the favorable solvent properties of lower-molecular weight ethers and alcohols. The word "Cellosolve" was registered in 1924 as a United States trademark by Carbide & Carbon Chemicals Corp. for "Solvents for Gums, Resins, Cellulose Esters, and the Like". The first one was ethyl cellosolve, with the name now generic for glycol ethers.

2-Methoxyethanol, or methyl cellosolve, is an organic compound with formula C
3H
8O
2 that is used mainly as a solvent. It is a clear, colorless liquid with an ether-like odor. It is in a class of solvents known as glycol ethers which are notable for their ability to dissolve a variety of different types of chemical compounds and for their miscibility with water and other solvents. It can be formed by the nucleophilic attack of methanol on protonated ethylene oxide followed by proton transfer:

Methyllithium is the simplest organolithium reagent with the empirical formula CH3Li. This s-block organometallic compound adopts an oligomeric structure both in solution and in the solid state. This highly reactive compound, invariably used as a solution in ethers, is a reagent in organic synthesis as well as organometallic chemistry. Operations involving methyllithium require anhydrous conditions, because the compound is highly reactive toward water. Oxygen and carbon dioxide are also incompatible with MeLi. Methyllithium is usually not prepared, but purchased as a solution in various ethers.
Methylcyclohexane is an organic compound with the molecular formula is CH3C6H11. Classified as saturated hydrocarbon, it is a colourless liquid with a faint odor. Methylcyclohexane is used as a solvent. It is mainly converted in naphtha reformers to toluene. Methylcyclohexane is also used in some correction fluids (such as White-Out) as a solvent.
N-Methylformamide (NMF) is a colorless, nearly odorless, organic compound and secondary amide with molecular formula CH3NHCHO, which is a liquid at room temperature. NMF is mainly used as a reagent in various organic syntheses with limited applications as a highly polar solvent.

Ferrocenium tetrafluoroborate is an organometallic compound with the formula [Fe(C5H5)2]BF4. This salt is composed of the cation [Fe(C5H5)2]+ and the tetrafluoroborate anion (BF−
4). The related hexafluorophosphate is also a popular reagent with similar properties. The cation is often abbreviated Fc+ or Cp2Fe+. The salt is deep blue in color and paramagnetic. Ferrocenium salts are sometimes used as one-electron oxidizing agents, and the reduced product, ferrocene, is inert and readily separated from ionic products. The ferrocene–ferrocenium couple is often used as a reference in electrochemistry. The standard potential of ferrocene-ferrocenium is 0.400 V vs. the normal hydrogen electrode (NHE) and is often assumed to be invariant between different solvents.
2-(2-Methoxyethoxy)ethanol, also known under trade names Methyl Carbitol, is an industrial solvent and is also commonly used as a Fuel System Icing Inhibitor (FSII) in jet fuels. It is a clear, colorless, hygroscopic liquid. Structurally it is an alcohol and an ether, with a formula CH3OCH2CH2OCH2CH2OH. At direct contact it causes drying of skin by leaching fats, and is mildly irritating to the eyes. It is flammable.

Methyl propionate, also known as methyl propanoate, is the organic compound with the molecular formula CH3CH2CO2CH3. It is a colorless liquid with a fruity, rum-like odor.

Methyl lactate, also known as lactic acid methyl ester, is the organic compound with the formula CH3CH(OH)CO2CH3. It is the methyl ester of lactic acid. A colorless liquid, it is the simplest chiral ester. Being naturally derived, it is readily available as a single enantiomer.













