Amyl nitrite is a chemical compound with the formula C5H11ONO. A variety of isomers are known, but they all feature an amyl group attached to the nitrite functional group. The alkyl group is unreactive and the chemical and biological properties are mainly due to the nitrite group. Like other alkyl nitrites, amyl nitrite is bioactive in mammals, being a vasodilator, which is the basis of its use as a prescription medicine. As an inhalant, it also has a psychoactive effect, which has led to its recreational use, with its smell being described as that of old socks or dirty feet. It was first documented in 1844 and came into medical use in 1867.
Perfume is a mixture of fragrant essential oils or aroma compounds (fragrances), fixatives and solvents, usually in liquid form, used to give the human body, animals, food, objects, and living-spaces an agreeable scent. Perfumes can be defined as substances that emit and diffuse a pleasant and fragrant odor. They consist of manmade mixtures of aromatic chemicals and essential oils. The 1939 Nobel Laureate for Chemistry, Leopold Ružička stated in 1945 that "right from the earliest days of scientific chemistry up to the present time, perfumes have substantially contributed to the development of organic chemistry as regards methods, systematic classification, and theory."
In organic chemistry, an alkyl group is an alkane missing one hydrogen. The term alkyl is intentionally unspecific to include many possible substitutions. An acyclic alkyl has the general formula of −CnH2n+1. A cycloalkyl group is derived from a cycloalkane by removal of a hydrogen atom from a ring and has the general formula −CnH2n−1. Typically an alkyl is a part of a larger molecule. In structural formulae, the symbol R is used to designate a generic (unspecified) alkyl group. The smallest alkyl group is methyl, with the formula −CH3.

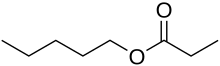
Propionic acid is a naturally occurring carboxylic acid with chemical formula CH
3CH
2CO
2H. It is a liquid with a pungent and unpleasant smell somewhat resembling body odor. The anion CH
3CH
2CO−
2 as well as the salts and esters of propionic acid are known as propionates or propanoates.

Amyl nitrate is the chemical compound with the formula CH3(CH2)4ONO2. This molecule consists of the 5-carbon amyl group attached to a nitrate functional group. It is the ester of amyl alcohol and nitric acid.
1-Pentanol,, is an organic compound with the formula CH3CH2CH2CH2CH2OH and is classified as a primary alcohol. It is a colourless liquid with a distinctive aroma. It is one of 8 isomeric alcohols with the formula C5H11OH. It is used as a solvent, a biological drying agent and in the synthesis of some fragrance compounds. It is also a common component of fusel alcohols, the undesirable byproducts of alcoholic fermentation.
ChapStick is a brand name of lip balm manufactured by Suave Brands Company and used in many countries worldwide. It is intended to help treat and prevent chapped lips, hence the name. Many varieties also include sunscreen in order to prevent sunburn.

Clobetasol propionate is a corticosteroid used to treat skin conditions such as eczema, contact dermatitis, seborrheic dermatitis, and psoriasis. It is applied to the skin as a cream, ointment, or shampoo. Use should be short term and only if other weaker corticosteroids are not effective. Use is not recommended in rosacea or perioral dermatitis.

Valeric acid or pentanoic acid is a straight-chain alkyl carboxylic acid with the chemical formula CH3(CH2)3COOH. Like other low-molecular-weight carboxylic acids, it has an unpleasant odor. It is found in the perennial flowering plant Valeriana officinalis, from which it gets its name. Its primary use is in the synthesis of its esters. Salts and esters of valeric acid are known as valerates or pentanoates. Volatile esters of valeric acid tend to have pleasant odors and are used in perfumes and cosmetics. Several, including ethyl valerate and pentyl valerate are used as food additives because of their fruity flavors.
Poppers is a slang term referring to recreational drugs belonging to the alkyl nitrite family of chemical compounds. When fumes from these substances are inhaled, they act as potent vasodilators, producing mild euphoria, warmth, and dizziness. Most effects have a rapid onset and are short-acting. Reported adverse effects include retinal toxicity and vision loss. As poppers include a broad range of chemical types, their legality differs across different jurisdictions. They are often packaged under the guise of room deodorizer, leather polish, nail polish remover, or videotape head cleaner to evade anti-drug laws.

Amyl acetate (pentyl acetate) is an organic compound and an ester with the chemical formula CH3COO[CH2]4CH3 and the molecular weight 130.19 g/mol. It is colorless and has a scent similar to bananas and apples. The compound is the condensation product of acetic acid and 1-pentanol. However, esters formed from other pentanol isomers (amyl alcohols), or mixtures of pentanols, are often referred to as amyl acetate. The symptoms of exposure to amyl acetate in humans are dermatitis, central nervous system depression, narcosis and irritation to the eyes and nose.

Pentyl is a five-carbon alkyl group or substituent with chemical formula -C5H11. It is the substituent form of the alkane pentane.

Amylmetacresol (AMC) is an antiseptic used to treat infections of the mouth and throat. It is used as an active pharmaceutical ingredient in Strepsils, Cēpacol, Gorpils, Cofsils and Lorsept throat lozenges, typically in combination with dichlorobenzyl alcohol, another antiseptic.
Heptanal or heptanaldehyde is an alkyl aldehyde. It is a colourless liquid with a strong fruity odor, which is used as precursor to components in perfumes and lubricants.
tert-Amyl methyl ether (TAME) is an ether used as a fuel oxygenate. TAME derives from C5 distillation fractions of naphtha. It has an ethereous odor. Unlike most ethers, it does not require a stabilizer as it does not form peroxides on storage.

JWH-203 (1-pentyl-3-(2-chlorophenylacetyl)indole) is an analgesic chemical from the phenylacetylindole family that acts as a cannabinoid agonist with approximately equal affinity at both the CB1 and CB2 receptors, having a Ki of 8.0 nM at CB1 and 7.0 nM at CB2. It was originally discovered by, and named after, John W. Huffman, but has subsequently been sold without his permission as an ingredient of synthetic cannabis smoking blends. Similar to the related 2'-methoxy compound JWH-250, the 2'-bromo compound JWH-249, and the 2'-methyl compound JWH-251, JWH-203 has a phenylacetyl group in place of the naphthoyl ring used in most aminoalkylindole cannabinoid compounds, and has the strongest in vitro binding affinity for the cannabinoid receptors of any compound in the phenylacetyl group.

APINACA (AKB48, N-(1-adamantyl)-1-pentyl-1H-indazole-3-carboxamide) is a drug that acts as a reasonably potent agonist for the cannabinoid receptors. It is a full agonist at CB1 with an EC50 of 142 nM and Ki of 3.24 nM (compared to the Ki of Δ9-THC at 28.35 nM and JWH-018 at 9.62 nM), while at CB2 it acts as a partial agonist with an EC50 of 141 nM and Ki of 1.68 nM (compared to the Ki of Δ9-THC at 37.82 nM and JWH-018 at 8.55 nM). Its pharmacological characterization has also been reported in a discontinued patent application. It had never previously been reported in the scientific or patent literature, and was first identified by laboratories in Japan in March 2012 as an ingredient in synthetic cannabis smoking blends, along with a related compound APICA. Structurally, it closely resembles cannabinoid compounds from a University of Connecticut patent, but with a simple pentyl chain on the indazole 1-position, and APINACA falls within the claims of this patent despite not being disclosed as an example.

Methyl propionate, also known as methyl propanoate, is an organic compound with the molecular formula CH3CH2CO2CH3. It is a colorless liquid with a fruity, rum-like odor.

6-Amyl-α-pyrone, also 6-pentyl-2-pyrone or 6PP, is an unsaturated lactone molecule. It contains two double bonds in the ring and a pentyl substituent at carbon adjacent to the ring oxygen. It is a colorless liquid which possesses characteristic coconut aroma, produced biologically by Trichoderma species. It is found in animal foods, peach, and heated beef.