In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.

A plum is a fruit of some species in Prunus subg. Prunus. Dried plums are most often called prunes, though in the United States they may be just labeled as 'dried plums', especially during the 21st century.

Prunus is a genus of trees and shrubs in the flowering plant family Rosaceae that includes plums, cherries, peaches, nectarines, apricots, and almonds. The genus has a cosmopolitan distribution, being native to the North American temperate regions, the neotropics of South America, and temperate and tropical regions of Asia and Africa, There are 340 accepted species. Many members of the genus are widely cultivated for their fruit and for decorative purposes. Prunus fruit are drupes, or stone fruits. The fleshy mesocarp surrounding the endocarp is edible while the endocarp itself forms a hard, inedible shell called the pyrena. This shell encloses the seed, which is edible in some species, but poisonous in many others. Besides being eaten off the hand, most Prunus fruit are also commonly used in processing, such as jam production, canning, drying, and the seeds for roasting.
1-Pentanol,, is an organic compound with the formula CH3CH2CH2CH2CH2OH and is classified as a primary alcohol. It is a colourless liquid with a distinctive aroma. It is one of 8 isomeric alcohols with the formula C5H11OH. It is used as a solvent, a biological drying agent and in the synthesis of some fragrance compounds. It is also a common component of fusel alcohols, the undesirable byproducts of alcoholic fermentation.
In chemistry, acetylation is an organic esterification reaction with acetic acid. It introduces an acetyl group into a chemical compound. Such compounds are termed acetate esters or simply acetates. Deacetylation is the opposite reaction, the removal of an acetyl group from a chemical compound.
Acetamide (systematic name: ethanamide) is an organic compound with the formula CH3CONH2. It is derived from acetic acid. It finds some use as a plasticizer and as an industrial solvent. The related compound N,N-dimethylacetamide (DMA) is more widely used, but it is not prepared from acetamide. Acetamide can be considered an intermediate between acetone, which has two methyl (CH3) groups either side of the carbonyl (CO), and urea which has two amide (NH2) groups in those locations. Acetamide is also a naturally occurring mineral with the IMA symbol: Ace.
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula −C4H9, derived from either of the two isomers (n-butane and isobutane) of butane.

Ethyl acetate is the organic compound with the formula CH3CO2CH2CH3, simplified to C4H8O2. This colorless liquid has a characteristic sweet smell and is used in glues, nail polish removers, and in the decaffeination process of tea and coffee. Ethyl acetate is the ester of ethanol and acetic acid; it is manufactured on a large scale for use as a solvent.

Methyl acetate, also known as MeOAc, acetic acid methyl ester or methyl ethanoate, is a carboxylate ester with the formula CH3COOCH3. It is a flammable liquid with a characteristically pleasant smell reminiscent of some glues and nail polish removers. Methyl acetate is occasionally used as a solvent, being weakly polar and lipophilic, but its close relative ethyl acetate is a more common solvent being less toxic and less soluble in water. Methyl acetate has a solubility of 25% in water at room temperature. At elevated temperature its solubility in water is much higher. Methyl acetate is not stable in the presence of strong aqueous bases or aqueous acids. Methyl acetate is not considered a VOC in the USA.
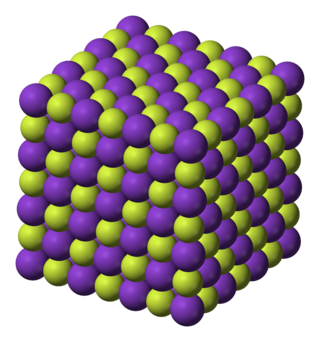
Potassium fluoride is the chemical compound with the formula KF. After hydrogen fluoride, KF is the primary source of the fluoride ion for applications in manufacturing and in chemistry. It is an alkali halide salt and occurs naturally as the rare mineral carobbiite. Solutions of KF will etch glass due to the formation of soluble fluorosilicates, although HF is more effective.

Liquid–liquid extraction, also known as solvent extraction and partitioning, is a method to separate compounds or metal complexes, based on their relative solubilities in two different immiscible liquids, usually water (polar) and an organic solvent (non-polar). There is a net transfer of one or more species from one liquid into another liquid phase, generally from aqueous to organic. The transfer is driven by chemical potential, i.e. once the transfer is complete, the overall system of chemical components that make up the solutes and the solvents are in a more stable configuration. The solvent that is enriched in solute(s) is called extract. The feed solution that is depleted in solute(s) is called the raffinate. Liquid-liquid extraction is a basic technique in chemical laboratories, where it is performed using a variety of apparatus, from separatory funnels to countercurrent distribution equipment called as mixer settlers. This type of process is commonly performed after a chemical reaction as part of the work-up, often including an acidic work-up.

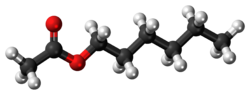
n-Butyl acetate is an organic compound with the formula CH3CO2(CH2)3CH3. A colorless, flammable liquid, it is the ester derived from n-butanol and acetic acid. It is found in many types of fruit, where it imparts characteristic flavors and has a sweet smell of banana or apple. It is used as an industrial solvent.

Propyl acetate, also known as propyl ethanoate, is an organic compound. Nearly 20,000 tons are produced annually for use as a solvent. This colorless liquid is known by its characteristic odor of pears. Due to this fact, it is commonly used in fragrances and as a flavor additive. It is formed by the esterification of acetic acid and propan-1-ol, often via Fischer–Speier esterification, with sulfuric acid as a catalyst and water produced as a byproduct.

Isoamyl acetate, also known as isopentyl acetate, is an ester formed from isoamyl alcohol and acetic acid, with the molecular formula . It is a colorless liquid that is only slightly soluble in water, but very soluble in most organic solvents. Isoamyl acetate has a strong odor which is described as similar to both banana and pear. Pure isoamyl acetate, or mixtures of isoamyl acetate, amyl acetate, and other flavors in ethanol may be referred to as banana oil or pear oil.

Propylene carbonate (often abbreviated PC) is an organic compound with the formula C4H6O3. It is a cyclic carbonate ester derived from propylene glycol. This colorless and odorless liquid is useful as a polar, aprotic solvent. Propylene carbonate is chiral, but is used as the racemic mixture in most contexts.

Basic beryllium acetate is the chemical compound with the formula Be4O(O2CCH3)6. This compound adopts a distinctive structure, but it has no applications and has been only lightly studied. It is a colourless solid that is soluble in organic solvents.

The Nozaki–Hiyama–Kishi reaction is a nickel/chromium coupling reaction forming an alcohol from the reaction of an aldehyde with an allyl or vinyl halide. In their original 1977 publication, Tamejiro Hiyama and Hitoshi Nozaki reported on a chromium(II) salt solution prepared by reduction of chromic chloride by lithium aluminium hydride to which was added benzaldehyde and allyl chloride:

Acetic acid, systematically named ethanoic acid, is an acidic, colourless liquid and organic compound with the chemical formula CH3COOH. Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. It has been used, as a component of vinegar, throughout history from at least the third century BC.

Extraction in chemistry is a separation process consisting of the separation of a substance from a matrix. The distribution of a solute between two phases is an equilibrium condition described by partition theory. This is based on exactly how the analyte moves from the initial solvent into the extracting solvent. The term washing may also be used to refer to an extraction in which impurities are extracted from the solvent containing the desired compound.

Prunus simonii, called apricot plum and Simon plum, is a tree in the genus Prunus. It was first described by Elie-Abel Carrière in 1872 and is native to Hebei province, China. The species is not known in a truly wild state. It has been important for breeding commercial plum cultivars from crosses with other species of the genus Prunus. The species is named for Gabriel Eugène Simon (1829–1896), a French botanist and diplomat who sent pits to the Paris Museum in the early 1860s while he was representing the French government in China. Beginning about 1881, the species became commonly known in the United States; having been introduced there from France.