
Indigo is a term used for a number of hues in the region of blue. The word comes from the ancient dye of the same name. The term "indigo" can refer to the color of the dye, various colors of fabric dyed with indigo dye, a spectral color, one of the seven colors of the rainbow as described by Newton, or a region on the color wheel, and can include various shades of blue, ultramarine, and green-blue. Since the web era, the term has also been used for various purple and violet hues identified as "indigo", based on use of the term "indigo" in HTML web page specifications.

Violet is the color of light at the short wavelength end of the visible spectrum. It is one of the seven colors that Isaac Newton labeled when dividing the spectrum of visible light in 1672. Violet light has a wavelength between approximately 380 and 435 nanometers. The color's name is derived from the Viola genus of flowers.

Purple is a color similar in appearance to violet light. In the RYB color model historically used in the arts, purple is a secondary color created by combining red and blue pigments. In the CMYK color model used in modern printing, purple is made by combining magenta pigment with either cyan pigment, black pigment, or both. In the RGB color model used in computer and television screens, purple is created by mixing red and blue light in order to create colors that appear similar to violet light.

Methyl isocyanate (MIC) is an organic compound with the molecular formula CH3NCO. Synonyms are isocyanatomethane and methyl carbylamine. Methyl isocyanate is an intermediate chemical in the production of carbamate pesticides (such as carbaryl, carbofuran, methomyl, and aldicarb). It has also been used in the production of rubbers and adhesives. As an extremely toxic and irritating compound, it is very hazardous to human health. MIC was the principal toxicant involved in the Bhopal gas disaster, which short-term killed 4,000–8,000 people and caused permanent injury and premature deaths to tens of thousands more. It is also a very potent lachrymatory agent.

Mauveine, also known as aniline purple and Perkin's mauve, was one of the first synthetic dyes. It was discovered serendipitously by William Henry Perkin in 1856 while he was attempting to synthesise the phytochemical quinine for the treatment of malaria. It is also among the first chemical dyes to have been mass-produced.
Methyl violet is a family of organic compounds that are mainly used as dyes. Depending on the number of attached methyl groups, the color of the dye can be altered. Its main use is as a purple dye for textiles and to give deep violet colors in paint and ink. It is also used as a hydration indicator for silica gel. Methyl violet 10B is also known as crystal violet and has medical uses.

Casuarina equisetifolia, commonly known as coastal she-oak, horsetail she-oak, ironwood,beach sheoak, beach casuarina, whistling tree or Australian pine is a species of flowering plant in the family Casuarinaceae and is native to Australia, New Guinea, Southeast Asia and India. It is a small to medium-sized, monoecious tree with scaly or furrowed bark on older specimens, drooping branchlets, the leaves reduced to scales in whorls of 7 or 8, the fruit 10–24 mm (0.4–0.9 in) long containing winged seeds (samaras) 6–8 mm (0.2–0.3 in) long.

Methyl orange is a pH indicator frequently used in titration because of its clear and distinct color variance at different pH values. Methyl orange shows red color in acidic medium and yellow color in basic medium. Because it changes color at the pKa of a mid strength acid, it is usually used in titration of strong acids in weak bases that reach the equivalence point at a pH of 3.1-4.4. Unlike a universal indicator, methyl orange does not have a full spectrum of color change, but it has a sharp end point. In a solution becoming less acidic, methyl orange changes from red to orange and, finally, to yellow—with the reverse process occurring in a solution of increasing acidity.
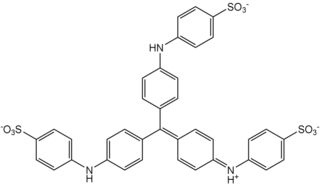
Methyl blue is a chemical compound with the molecular formula C37H27N3Na2O9S3. It is used as a stain in histology, and stains collagen blue in tissue sections. It can be used in some differential staining techniques such as Mallory's connective tissue stain and Gömöri trichrome stain, and can be used to mediate electron transfer in microbial fuel cells. Fungal cell walls are also stained by methyl blue.
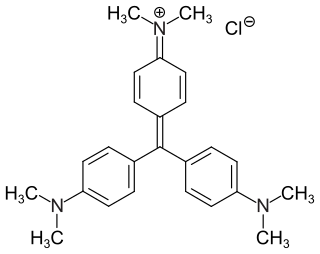
Crystal violet or gentian violet, also known as methyl violet 10B or hexamethyl pararosaniline chloride, is a triarylmethane dye used as a histological stain and in Gram's method of classifying bacteria. Crystal violet has antibacterial, antifungal, and anthelmintic (vermicide) properties and was formerly important as a topical antiseptic. The medical use of the dye has been largely superseded by more modern drugs, although it is still listed by the World Health Organization.
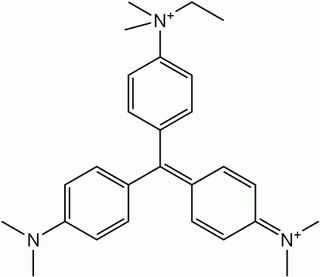
The dye Ethyl Green (C.I. 42590; C27H35BrClN3) is a triarylmethane dye. It is soluble in water.
IARC group 2B substances, mixtures and exposure circumstances are those that have been classified as "possibly carcinogenic to humans" by the International Agency for Research on Cancer (IARC) as This category is used when there is limited evidence of carcinogenicity in humans and less than sufficient evidence of carcinogenicity in experimental animals. It may also be used when there is insufficient evidence of carcinogenicity in humans but sufficient evidence in experimental animals. In some cases, an agent, mixture, or exposure circumstance with inadequate evidence of carcinogenicity in humans but limited evidence in experimental animals, combined with supporting evidence from other relevant data, may be included in this group.

Periodic acid–Schiff (PAS) is a staining method used to detect polysaccharides such as glycogen, and mucosubstances such as glycoproteins, glycolipids and mucins in tissues. The reaction of periodic acid oxidizes the vicinal diols in these sugars, usually breaking up the bond between two adjacent carbons not involved in the glycosidic linkage or ring closure in the ring of the monosaccharide units that are parts of the long polysaccharides, and creating a pair of aldehydes at the two free tips of each broken monosaccharide ring. The oxidation condition has to be sufficiently regulated so as to not oxidize the aldehydes further. These aldehydes then react with the Schiff reagent to give a purple-magenta color. A suitable basic stain is often used as a counterstain.

Artocarpus odoratissimus is a species of flowering plant in the Moraceae family. It is a commonly called marang, madang, timadang, terap, tarap, kiran, green pedalai, or johey oak. It is native to Borneo, Palawan, and Mindanao Island, and is closely related to the jackfruit, cempedak, and breadfruit trees which all belong to the same genus, Artocarpus.

Methyl red (2-(N,N-dimethyl-4-aminophenyl) azobenzenecarboxylic acid), also called C.I. Acid Red 2, is an indicator dye that turns red in acidic solutions. It is an azo dye, and is a dark red crystalline powder. Methyl red is a pH indicator; it is red in pH under 4.4, yellow in pH over 6.2, and orange in between, with a pKa of 5.1. Murexide and methyl red are investigated as promising enhancers of sonochemical destruction of chlorinated hydrocarbon pollutants. Methyl red is classed by the IARC in group 3 - unclassified as to carcinogenic potential in humans.
Triarylmethane dyes are synthetic organic compounds containing triphenylmethane backbones. As dyes, these compounds are intensely colored. They are produced industrially as dyes.
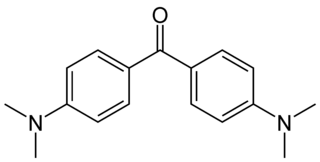
Michler's ketone is an organic compound with the formula of [(CH3)2NC6H4]2CO. This electron-rich derivative of benzophenone is an intermediate in the production of dyes and pigments, for example Methyl violet. It is also used as a photosensitizer. It is named after the German chemist Wilhelm Michler.

Calcofluor-white or CFW is a fluorescent blue dye used in biology and textiles. It binds to 1-3 beta and 1-4 beta polysaccharides of chitin and cellulose that are present in cell walls on fungi, plants, and algae.

Azolla pinnata is a species of fern known by several common names, including mosquitofern, feathered mosquitofern and water velvet. It is native to much of Africa, Asia and parts of Australia. It is an aquatic plant, it is found floating upon the surface of the water. It grows in quiet and slow-moving water bodies because swift currents and waves break up the plant. At maximum growth rate, it can double its biomass in 1.9 days, with most strains attaining such growth within a week under optimal conditions.

Methyl violet 6B is a violet triarylmethane dye from the group of cationic dyes and an essential component of C.I. Basic Violet 1. The compound is sometimes equated with methyl violet in the literature.

















