
Methyl isocyanate (MIC) is an organic compound with the molecular formula CH3NCO. Synonyms are isocyanatomethane and methyl carbylamine. Methyl isocyanate is an intermediate chemical in the production of carbamate pesticides and Haffmann Bromamide Degradation (such as carbaryl, carbofuran, methomyl, and aldicarb). It has also been used in the production of rubbers and adhesives. As an extremely toxic and irritating compound, it is very hazardous to human health. MIC was the principal toxicant involved in the Bhopal gas disaster, which short-term killed 4,000–8,000 people and caused permanent injury and premature deaths to approximately 15,000-20,000. It is also a very potent lachrymatory agent.

N,N-Dimethylaniline (DMA) is an organic chemical compound, a substituted derivative of aniline. It is a tertiary amine, featuring a dimethylamino group attached to a phenyl group. This oily liquid is colourless when pure, but commercial samples are often yellow. It is an important precursor to dyes such as crystal violet.

Aniline is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans.

Mauveine, also known as aniline purple and Perkin's mauve, was one of the first synthetic dyes. It was discovered serendipitously by William Henry Perkin in 1856 while he was attempting to synthesise the phytochemical quinine for the treatment of malaria. It is also among the first chemical dyes to have been mass-produced.
Methyl violet is a family of organic compounds that are mainly used as dyes. Depending on the number of attached methyl groups, the color of the dye can be altered. Its main use is as a purple dye for textiles and to give deep violet colors in paint and ink. It is also used as a hydration indicator for silica gel. Methyl violet 10B is also known as crystal violet and has medical uses.

Azo compounds are organic compounds bearing the functional group diazenyl.

Methyl orange is a pH indicator frequently used in titration because of its clear and distinct color variance at different pH values. Methyl orange shows red color in acidic medium and yellow color in basic medium. Because it changes color at the pKa of a mid strength acid, it is usually used in titration of strong acids in weak bases that reach the equivalence point at a pH of 3.1-4.4. Unlike a universal indicator, methyl orange does not have a full spectrum of color change, but it has a sharp end point. In a solution becoming less acidic, methyl orange changes from red to orange and, finally, to yellow—with the reverse process occurring in a solution of increasing acidity.
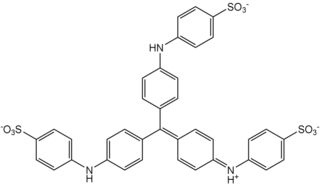
Methyl blue is a chemical compound with the molecular formula C37H27N3Na2O9S3. It is used as a stain in histology, and stains collagen blue in tissue sections. It can be used in some differential staining techniques such as Mallory's connective tissue stain and Gömöri trichrome stain, and can be used to mediate electron transfer in microbial fuel cells. Fungal cell walls are also stained by methyl blue.

Methyl yellow, or C.I. 11020, is an organic compound with the formula C6H5N2C6H4N(CH3)2. It is an azo dye derived from dimethylaniline. It is a yellow solid. According to X-ray crystallography, the C14N3 core of the molecule is planar.
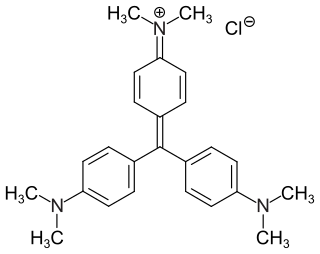
Crystal violet or gentian violet, also known as methyl violet 10B or hexamethyl pararosaniline chloride, is a triarylmethane dye used as a histological stain and in Gram's method of classifying bacteria. Crystal violet has antibacterial, antifungal, and anthelmintic (vermicide) properties and was formerly important as a topical antiseptic. The medical use of the dye has been largely superseded by more modern drugs, although it is still listed by the World Health Organization.
IARC group 2B substances, mixtures and exposure circumstances are those that have been classified as "possibly carcinogenic to humans" by the International Agency for Research on Cancer (IARC) as This category is used when there is limited evidence of carcinogenicity in humans and less than sufficient evidence of carcinogenicity in experimental animals. It may also be used when there is insufficient evidence of carcinogenicity in humans but sufficient evidence in experimental animals. In some cases, an agent, mixture, or exposure circumstance with inadequate evidence of carcinogenicity in humans but limited evidence in experimental animals, combined with supporting evidence from other relevant data, may be included in this group.
IARC group 3 substances, chemical mixtures and exposure circumstances are those that can not be classified in regard to their carcinogenicity to humans by the International Agency for Research on Cancer (IARC). This category is used most commonly for agents, mixtures and exposure circumstances for which the level of evidence of carcinogenicity is inadequate in humans and inadequate or limited in experimental animals. Exceptionally, agents (mixtures) for which the evidence of carcinogenicity is inadequate in humans, but sufficient in experimental animals may be placed in this category when there is strong evidence that the mechanism of carcinogenicity in experimental animals does not operate in humans. Agents, mixtures and exposure circumstances that do not fall into any other group are also placed in this category.

Methyl red (2-(N,N-dimethyl-4-aminophenyl) azobenzenecarboxylic acid), also called C.I. Acid Red 2, is an indicator dye that turns red in acidic solutions. It is an azo dye, and is a dark red crystalline powder. Methyl red is a pH indicator; it is red in pH under 4.4, yellow in pH over 6.2, and orange in between, with a pKa of 5.1. Murexide and methyl red are investigated as promising enhancers of sonochemical destruction of chlorinated hydrocarbon pollutants. Methyl red is classed by the IARC in group 3 - unclassified as to carcinogenic potential in humans.

Amitraz is a non-systemic acaricide and insecticide and has also been described as a scabicide. It was first synthesized by the Boots Co. in England in 1969. Amitraz has been found to have an insect repellent effect, works as an insecticide and also as a pesticide synergist. Its effectiveness is traced back on alpha-adrenergic agonist activity, interaction with octopamine receptors of the central nervous system and inhibition of monoamine oxidases and prostaglandin synthesis. Therefore, it leads to overexcitation and consequently paralysis and death in insects. Because amitraz is less harmful to mammals, amitraz is among many other purposes best known as insecticide against mite- or tick-infestation of dogs. It is also widely used in the beekeeping industry as a control for the Varroa destructor mite, although there are recent reports of resistance.

Sulfanilic acid (4-aminobenzenesulfonic acid) is an organic compound with the formula H3NC6H4SO3. It is an off-white solid. It is a zwitterion, which explains its high melting point. It is a common building block in organic chemistry.
Triarylmethane dyes are synthetic organic compounds containing triphenylmethane backbones. As dyes, these compounds are intensely colored. They are produced industrially as dyes.
Azobenzene reductase also known as azoreductase (EC 1.7.1.6) is an enzyme that catalyzes the chemical reaction:
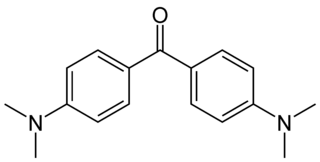
Michler's ketone is an organic compound with the formula of [(CH3)2NC6H4]2CO. This electron-rich derivative of benzophenone is an intermediate in the production of dyes and pigments, for example Methyl violet. It is also used as a photosensitizer. It is named after the German chemist Wilhelm Michler.
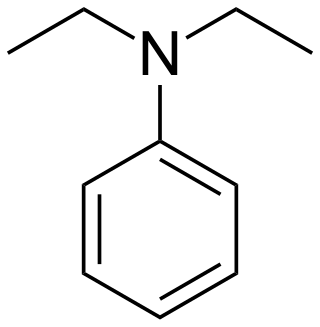
Diethylaniline is the organic compound with the molecular formula (C2H5)2NC6H5. It is a colorless liquid but commercial samples are often yellow. It is a precursor to several dyes and other commercial products.

Methyl violet 2B (Tetramethylparosanilinium chloride, 4,4′-[(4-imino-2,5-cyclohexadien-1-yliden)methylen]bis(N,N-dimethylaniline)hydrochloride) is a violet triarylmethane dye from the group of cationic dyes and an essential component of C.I. Basic Violet 1 (trivial name methyl violet). Methyl violets are mixtures of tetramethyl (2B), pentamethyl (6B) and hexamethyl (10B) pararosanilins.
















