
Arsine (IUPAC name: arsane) is an inorganic compound with the formula AsH3. This flammable, pyrophoric, and highly toxic pnictogen hydride gas is one of the simplest compounds of arsenic. Despite its lethality, it finds some applications in the semiconductor industry and for the synthesis of organoarsenic compounds. The term arsine is commonly used to describe a class of organoarsenic compounds of the formula AsH3−xRx, where R = aryl or alkyl. For example, As(C6H5)3, called triphenylarsine, is referred to as "an arsine".

Valence shell electron pair repulsion (VSEPR) theory is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gillespie-Nyholm theory after its two main developers, Ronald Gillespie and Ronald Nyholm.
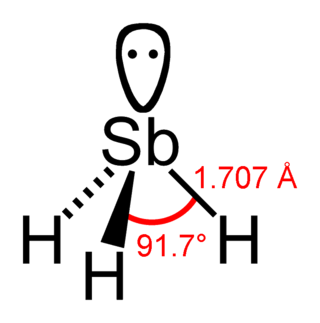
Stibine (IUPAC name: stibane) is a chemical compound with the formula SbH3. A pnictogen hydride, this colourless, highly toxic gas is the principal covalent hydride of antimony, and a heavy analogue of ammonia. The molecule is pyramidal with H–Sb–H angles of 91.7° and Sb–H distances of 170.7 pm (1.707 Å). The smell of this compound from usual sources (like from reduction of antimony compounds) is reminiscent of arsine, id est garlic-like.

Germane is the chemical compound with the formula GeH4, and the germanium analogue of methane. It is the simplest germanium hydride and one of the most useful compounds of germanium. Like the related compounds silane and methane, germane is tetrahedral. It burns in air to produce GeO2 and water. Germane is a group 14 hydride.

Vinylsilane refers to an organosilicon compound with chemical formula CH2=CHSiH3. It is a derivative of silane (SiH4). The compound, which is a colorless gas, is mainly of theoretical interest.

Aluminium hydride is an inorganic compound with the formula AlH3. Alane and its derivatives are part of a family of common reducing reagents in organic synthesis based around group 13 hydrides. In solution—typically in ethereal solvents such tetrahydrofuran or diethyl ether—aluminium hydride forms complexes with Lewis bases, and reacts selectively with particular organic functional groups, and although it is not a reagent of choice, it can react with carbon-carbon multiple bonds. Given its density, and with hydrogen content on the order of 10% by weight, some forms of alane are, as of 2016, active candidates for storing hydrogen and so for power generation in fuel cell applications, including electric vehicles. As of 2006 it was noted that further research was required to identify an efficient, economical way to reverse the process, regenerating alane from spent aluminium product.
The proton affinity of an anion or of a neutral atom or molecule is the negative of the enthalpy change in the reaction between the chemical species concerned and a proton in the gas phase:
Phenylsilane, also known as silylbenzene, a colorless liquid, is one of the simplest organosilanes with the formula C6H5SiH3. It is structurally related to toluene, with a silyl group replacing the methyl group. Both of these compounds have similar densities and boiling points due to these similarities. Phenylsilane is soluble in organic solvents.
Trisilane is the silane with the formula H2Si(SiH3)2. A liquid at standard temperature and pressure, it is a silicon analogue of propane. In contrast with propane, however, trisilane ignites spontaneously in air.
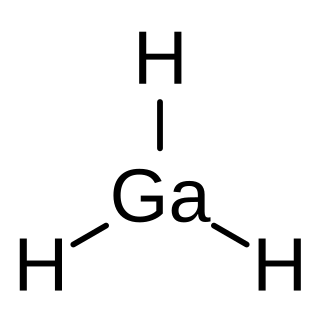
Gallane, also systematically named trihydridogallium, is an inorganic compound of gallium with the chemical formula GaH
3. It is a photosensitive, colourless gas that cannot be concentrated in pure form. Gallane is both the simplest member of the gallanes, and the prototype of the monogallanes. It has no economic uses, and is only intentionally produced for academic reasons.
Boron monofluoride or fluoroborylene is a chemical compound with the formula BF, one atom of boron and one of fluorine. It is an unstable gas, but it is a stable ligand on transition metals, in the same way as carbon monoxide. It is a subhalide, containing fewer than the normal number of fluorine atoms, compared with boron trifluoride. It can also be called a borylene, as it contains boron with two unshared electrons. BF is isoelectronic with carbon monoxide and dinitrogen; each molecule has 14 electrons.

Indium trihydride is an inorganic compound with the chemical formula. It has been observed in matrix isolation and laser ablation experiments. Gas phase stability has been predicted. The infrared spectrum was obtained in the gas phase by laser ablation of indium in presence of hydrogen gas InH3 is of no practical importance.
Thallane is an inorganic compound with the empirical chemical formula TlH3. It has not yet been obtained in bulk, hence its bulk properties remain unknown. However, molecular thallane has been isolated in solid gas matrices. Thallane is mainly produced for academic purposes.
Titanium(IV) hydride is an inorganic compound with the empirical chemical formula TiH
4. It has not yet been obtained in bulk, hence its bulk properties remain unknown. However, molecular titanium(IV) hydride has been isolated in solid gas matrices. The molecular form is a colourless gas, and very unstable toward thermal decomposition. As such the compound is not well characterised, although many of its properties have been calculated via computational chemistry.
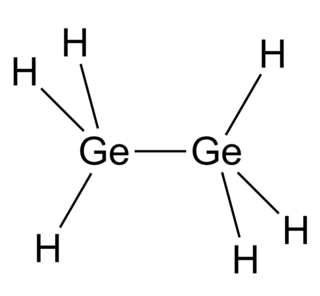
Digermane is an inorganic compound with the chemical formula Ge2H6. One of the few hydrides of germanium, it is a colourless liquid. Its molecular geometry is similar to ethane.
The dehydrogenative coupling of silanes is a reaction type for the formation of Si-Si bonds. Although never commercialized, the reaction has been demonstrated for the synthesis of certain disilanes as well as polysilanes. These reactions generally require catalysts.

A silanide is a chemical compound containing an anionic silicon(IV) centre, the parent ion being SiH−3. The hydrogen atoms can also be substituted to produce more complex derivative anions such as tris(trimethylsilyl)silanide (hypersilyl), tris(tert-butyl)silanide, tris(pentafluoroethyl)silanide, or triphenylsilanide. The simple silanide ion can also be called trihydridosilanide or silyl hydride.
An arsinide, arsanide, dihydridoarsenate(1−) or arsanyl compound is a chemical derivative of arsine, where one hydrogen atom is replaced with a metal or cation. The arsinide ion has formula AsH−2. It can be considered as a ligand with name arsenido or arsanido. Few chemists study arsanyl compounds, as they are both toxic and unstable. The IUPAC names are arsanide and dihydridoarsenate(1−). For the ligand the name is arsanido. The neutral −AsH2 group is termed arsanyl.
Germyl, trihydridogermanate(1-), trihydrogermanide, trihydridogermyl or according to IUPAC Red Book: germanide is an anion containing germanium bounded with three hydrogens, with formula GeH−3. Germyl is the IUPAC term for the –GeH3 group. For less electropositive elements the bond can be considered covalent rather than ionic as "germanide" indicates. Germanide is the base for germane when it loses a proton.
Neodymium(III) hydride is an inorganic compound composed of neodymium and hydrogen with a chemical formula NdH3. In this compound, the neodymium atom is in the +3 oxidation state and the hydrogen atoms are -1. It is highly reactive.













