![<span class="mw-page-title-main">Silicone</span> Family of polymers of the repeating form [R2Si–O–SiR2]](https://upload.wikimedia.org/wikipedia/commons/thumb/3/37/Caulking.jpg/320px-Caulking.jpg)
In organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane. They are typically colorless oils or rubber-like substances. Silicones are used in sealants, adhesives, lubricants, medicine, cooking utensils, thermal insulation, and electrical insulation. Some common forms include silicone oil, grease, rubber, resin, and caulk.

Trichlorosilane (TCS) is an inorganic compound with the formula HCl3Si. It is a colourless, volatile liquid. Purified trichlorosilane is the principal precursor to ultrapure silicon in the semiconductor industry. In water, it rapidly decomposes to produce a siloxane polymer while giving off hydrochloric acid. Because of its reactivity and wide availability, it is frequently used in the synthesis of silicon-containing organic compounds.
Chloromethane, also called methyl chloride, Refrigerant-40, R-40 or HCC 40, is an organic compound with the chemical formula CH3Cl. One of the haloalkanes, it is a colorless, sweet-smelling, flammable gas. Methyl chloride is a crucial reagent in industrial chemistry, although it is rarely present in consumer products, and was formerly utilized as a refrigerant. Most chloromethane is biogenic.

Polydimethylsiloxane (PDMS), also known as dimethylpolysiloxane or dimethicone, is a silicone polymer with a wide variety of uses, from cosmetics to industrial lubrication and passive daytime radiative cooling.
Neodymium(III) chloride or neodymium trichloride is a chemical compound of neodymium and chlorine with the formula NdCl3. This anhydrous compound is a mauve-colored solid that rapidly absorbs water on exposure to air to form a purple-colored hexahydrate, NdCl3·6H2O. Neodymium(III) chloride is produced from minerals monazite and bastnäsite using a complex multistage extraction process. The chloride has several important applications as an intermediate chemical for production of neodymium metal and neodymium-based lasers and optical fibers. Other applications include a catalyst in organic synthesis and in decomposition of waste water contamination, corrosion protection of aluminium and its alloys, and fluorescent labeling of organic molecules (DNA).
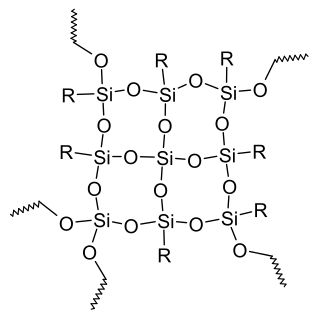
Silicone resins are a type of silicone material which is formed by branched, cage-like oligosiloxanes with the general formula of RnSiXmOy, where R is a non-reactive substituent, usually methyl or phenyl, and X is a functional group: hydrogen, hydroxyl, chlorine or alkoxy. These groups are further condensed in many applications, to give highly crosslinked, insoluble polysiloxane networks.

In organosilicon chemistry, a siloxane is an organic compound containing a functional group of two silicon atoms bound to an oxygen atom: Si−O−Si. The parent siloxanes include the oligomeric and polymeric hydrides with the formulae H[OSiH2]nOH and [OSiH2]n. Siloxanes also include branched compounds, the defining feature of which is that each pair of silicon centres is separated by one oxygen atom. The siloxane functional group forms the backbone of silicones [−R2Si−O−SiR2−]n, the premier example of which is polydimethylsiloxane (PDMS). The functional group R3SiO− is called siloxy. Siloxanes are manmade and have many commercial and industrial applications because of the compounds’ hydrophobicity, low thermal conductivity, and high flexibility.
In inorganic chemistry, chlorosilanes are a group of reactive, chlorine-containing chemical compounds, related to silane and used in many chemical processes. Each such chemical has at least one silicon-chlorine bond. Trichlorosilane is produced on the largest scale. The parent chlorosilane is silicon tetrachloride.

Trimethylsilyl chloride, also known as chlorotrimethylsilane is an organosilicon compound, with the formula (CH3)3SiCl, often abbreviated Me3SiCl or TMSCl. It is a colourless volatile liquid that is stable in the absence of water. It is widely used in organic chemistry.

Organosilicon chemistry is the study of organometallic compounds containing carbon–silicon bonds, to which they are called organosilicon compounds. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an inorganic compound.
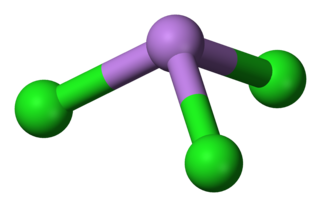
Arsenic trichloride is an inorganic compound with the formula AsCl3, also known as arsenous chloride or butter of arsenic. This poisonous oil is colourless, although impure samples may appear yellow. It is an intermediate in the manufacture of organoarsenic compounds.

Hexamethyldisiloxane (HMDSO or MM) is an organosilicon compound with the formula O[Si(CH3)3]2. This volatile colourless liquid is used as a solvent and as a reagent in organic synthesis. It is prepared by the hydrolysis of trimethylsilyl chloride. The molecule is the protypical disiloxane and resembles a subunit of polydimethylsiloxane.
Hydrosilanes are tetravalent silicon compounds containing one or more Si-H bond. The parent hydrosilane is silane (SiH4). Commonly, hydrosilane refers to organosilicon derivatives. Examples include phenylsilane (PhSiH3) and triethoxysilane ((C2H5O)3SiH). Polymers and oligomers terminated with hydrosilanes are resins that are used to make useful materials like caulks.
Trimethylsilanol (TMS) is an organosilicon compound with the formula (CH3)3SiOH. The Si centre bears three methyl groups and one hydroxyl group. It is a colourless volatile liquid.
Dimethyldichlorosilane is a tetrahedral organosilicon compound with the formula Si(CH3)2Cl2. At room temperature it is a colorless liquid that readily reacts with water to form both linear and cyclic Si-O chains. Dimethyldichlorosilane is made on an industrial scale as the principal precursor to dimethylsilicone and polysilane compounds.
The direct process, also called the direct synthesis, Rochow process, and Müller-Rochow process is the most common technology for preparing organosilicon compounds on an industrial scale. It was first reported independently by Eugene G. Rochow and Richard Müller in the 1940s.
In organosilicon chemistry, polysilazanes are polymers in which silicon and nitrogen atoms alternate to form the basic backbone. Since each silicon atom is bound to two separate nitrogen atoms and each nitrogen atom to two silicon atoms, both chains and rings of the formula [R2Si−NR]n occur. R can be hydrogen atoms or organic substituents. If all substituents R are hydrogen atoms, the polymer is designated as perhydropolysilazane, polyperhydridosilazane, or inorganic polysilazane ([H2Si−NH]n). If hydrocarbon substituents are bound to the silicon atoms, the polymers are designated as Organopolysilazanes. Molecularly, polysilazanes [R2Si−NH]n are isoelectronic with and close relatives to polysiloxanes [R2Si−O]n (silicones).

Polysilanes are organosilicon compounds with the formula (R2Si)n. They are relatives of traditional organic polymers but their backbones are composed of silicon atoms. They exhibit distinctive optical and electrical properties. They are mainly used as precursors to silicon carbide. The simplest polysilane would be (SiH2)n, which is mainly of theoretical, not practical interest.
In organosilicon chemistry, polysilynes are chemical compounds with the formula [RSi]n, where R can be hydrogen, or organyl. Although their name suggests a relationship to alkynes, polysilynes are a class of silicon-based random network polymers primarily composed of tetrahedral silicon atoms, each connected to one hydrogen or carbon and three Si atoms. These compounds are prepared by Wurtz coupling of alkyltrichlorosilanes :





![<span class="mw-page-title-main">Silicone</span> Family of polymers of the repeating form [R2Si–O–SiR2]](https://upload.wikimedia.org/wikipedia/commons/thumb/3/37/Caulking.jpg/320px-Caulking.jpg)





