
Azo compounds are compounds bearing the functional group diazenyl R−N=N−R′, in which R and R′ can be either aryl or alkyl.

Acenaphthene is a polycyclic aromatic hydrocarbon (PAH) consisting of naphthalene with an ethylene bridge connecting positions 1 and 8. It is a colourless solid. Coal tar consists of about 0.3% of this compound.
An azo coupling is an organic reaction between a diazonium compound and another aromatic compound that produces an azo compound. In this electrophilic aromatic substitution reaction, the aryldiazonium cation is the electrophile and the activated arene is a nucleophile. In most cases, including the examples below, the diazonium compound is also aromatic.
The Bucherer reaction in organic chemistry is the reversible conversion of a naphthol to a naphthylamine in the presence of ammonia and sodium bisulfite. The reaction is widely used in the synthesis of dye precursors aminonaphthalenesulfonic acids.
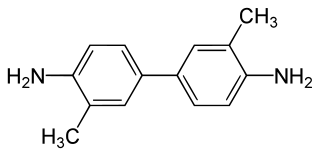
2-Tolidine (orthotolidine, o-tolidine; not to be confused with o-toluidine) is an organic compound with the chemical formula (C6H4(CH3)NH2)2. Several isomers are known; the 3-tolidine derivative is also important commercially. It is a colorless compound although commercial samples are often colored. It is slightly soluble in water. It forms salts with acids, such as the hydrochloride, which is commercially available.

Sulfanilic acid (4-aminobenzenesulfonic acid) is an organic compound with the formula H3NC6H4SO3. It is an off-white solid. It is a zwitterion, which explains its high melting point. It is a common building block in organic chemistry.
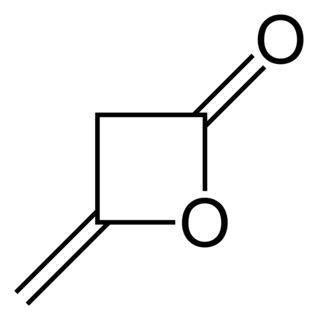
Diketene is an organic compound with the molecular formula C4H4O2, and which is sometimes written as (CH2CO)2. It is formed by dimerization of ketene, H2C=C=O. Diketene is a member of the oxetane family. It is used as a reagent in organic chemistry. It is a colorless liquid.

2-Naphthol, or β-naphthol, is a fluorescent colorless (or occasionally yellow) crystalline solid with the formula C10H7OH. It is an isomer of 1-naphthol, differing by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol, but more reactive. Both isomers are soluble in simple alcohols, ethers, and chloroform. 2-Naphthol is a widely used intermediate for the production of dyes and other compounds.
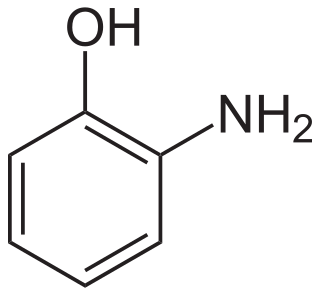
2-Aminophenol is an organic compound with the formula C6H7NO. Along with its isomer 4-aminophenol, it is an amphoteric molecule and a reducing agent. It is a useful reagent for the synthesis of dyes and heterocyclic compounds. Reflecting its slight hydrophilic character, white powder is moderately soluble in alcohols and can be recrystallized from hot water.

3,3'-Dichlorobenzidine is an organic compound with the formula (C6H3Cl(NH2))2. The pure compound is pale yellow, but commercial samples are often colored. It is barely soluble in water and is often supplied as a wet paste. It is widely used in the production of diarylide yellow pigments used in the production of printing inks. Its use in the production of dyes has been largely discontinued because of concerns about carcinogenicity.
1-Naphthol, or α-naphthol, is a fluorescent organic compound with the formula C10H7OH. It is a white solid. It is an isomer of 2-naphthol differing by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol, with the hydroxyl group being more reactive than in the phenols. Both isomers are soluble in simple alcohols, ethers, and chloroform. They are precursors to a variety of useful compounds. Naphthols (both 1 and 2 isomers) are used as biomarkers for livestock and humans exposed to polycyclic aromatic hydrocarbons.

Naphthol Green B is a coordination complex of iron that is used as a dye.

A rylene dye is a dye based on the rylene framework of naphthalene units linked in peri-positions. In homologues additional naphthalene units are added, forming compounds — or poly(peri-naphthalene)s — such as perylene, terrylene and quarterrylene.

Naphthionic acid is an organic compound with the formula C10H6(SO3H)(NH2). It is one of several aminonaphthalenesulfonic acids, derivatives of naphthalene containing both amine and sulfonic acid functional groups. It is a white solid, although commercial samples can appear gray. It is used in the synthesis of azo dyes such as Rocceline (a. k. a. Solid Red A), during which the amino group of the acid (in the form of a salt) is diazotated and then coupled with, in the case mentioned, β-naphthol. It is prepared by treating 1-aminonaphthalene with sulfuric acid.

Acetoacetanilide is an organic compound with the formula CH3C(O)CH2C(O)NHC6H5. It is the acetoacetamide derivative of aniline. It is a white solid that is poorly soluble in water. It and many related compounds (prepared from various aniline derivatives) are used in the production of organic pigments called arylide yellows.
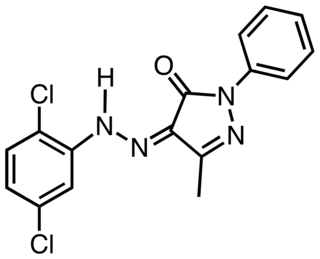
Pigment Yellow 10 is an organic compound that is classified as a Monoazopyrazolone pigment. It is used as a yellow colorant, notably as yellow road marking on highways in the US. The compound is synthesized by coupling the diazonium salt derived from dichloroaniline with the pyrazolone.
Pigment Red 190, also called Vat Red 29, is a synthetic organic compound that is used both as a pigment and as a vat dye. Although structurally a derivative of perylene, it is produced from acenaphthene.

Perylenetetracarboxylic dianhydride (PTCDA) is an organic dye molecule and an organic semiconductor. It is used as a precursor to a class of molecules known as Rylene dyes, which are useful as pigments and dyes. It is a dark red solid with low solubility in aromatic solvents. The compound has attracted much interest as an organic semiconductor.
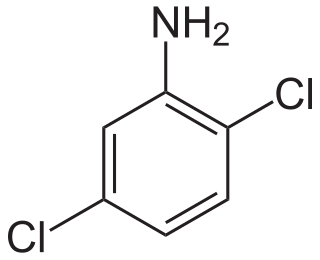
2,5-Dichloroaniline is an organic compound with the formula C6H3Cl2NH2. One of six isomers of dichloroaniline, it is a colorless solid that is insoluble in water. It is produced by hydrogenation of 1,4-dichloro-2-nitrobenzene. It is a precursor to dyes and pigments, e.g., Pigment Yellow 10.

3-Hydroxy-2-naphthoic acid is an organic compound with the formula C10H6(OH)(CO2H). It is one of the several carboxylic acids derived from 2-naphthol. It is a common precursor to azo dyes and pigments. It is prepared by carboxylation of 2-naphthol via the Kolbe–Schmitt reaction.


















