
Hair coloring, or hair dyeing, is the practice of changing the color of the hair on humans' heads. The main reasons for this are cosmetic: to cover gray or white hair, to alter hair to create a specific look, to change a color to suit preference or to restore the original hair color after it has been discolored by hairdressing processes or sun bleaching.

Azo compounds are organic compounds bearing the functional group diazenyl.

Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl and substituted aryl groups. They are a commercially important family of azo compounds, i.e. compounds containing the C-N=N-C linkage. Azo dyes are synthetic dyes and do not occur naturally. Most azo dyes contain only one azo group but there are some that contain two or three azo groups, called "diazo dyes" and "triazo dyes" respectively. Azo dyes comprise 60-70% of all dyes used in food and textile industries. Azo dyes are widely used to treat textiles, leather articles, and some foods. Chemically related derivatives of azo dyes include azo pigments, which are insoluble in water and other solvents.

Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group [R−N+≡N]X− where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide.
In organic chemistry, an azo coupling is an organic reaction between a diazonium compound and another aromatic compound that produces an azo compound. In this electrophilic aromatic substitution reaction, the aryldiazonium cation is the electrophile and the activated carbon act as a nucleophile. In most cases, including the examples below, the diazonium compound is also aromatic.
The Herz reaction, named after the chemist Richard Herz, is the chemical conversion of an aniline to the benzodithiazolium salt by its reaction with disulfur dichloride. The salt is called a Herz salt. Hydrolysis of this Herz salt give the corresponding sodium thiolate, which can be further converted to the 2-aminothiophenol.
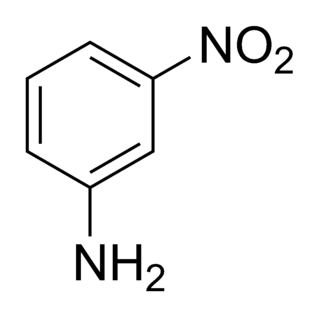
3-Nitroaniline is an organic compound with the formula H2NC6H4NO2. A yellow solid, it is a derivative of aniline, carrying a nitro functional group in position 3. It is an isomer of 2-nitroaniline and 4-nitroaniline. It is used as a precursor to dyes.
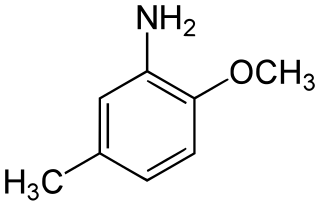
para-Cresidine is an organic compound with the formula CH3OC6H3(CH3)NH2. It is a white solid that is soluble in organic solvents. The compound features both amine and methoxy functional groups. It is used as an intermediate in preparation of dyes and pigments.
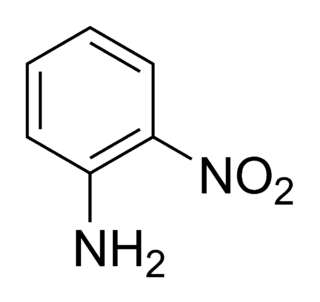
2-Nitroaniline is an organic compound with the formula H2NC6H4NO2. It is a derivative of aniline, carrying a nitro functional group in position 2. It is mainly used as a precursor to o-phenylenediamine.

4-Nitroaniline, p-nitroaniline or 1-amino-4-nitrobenzene is an organic compound with the formula C6H6N2O2. A yellow solid, it is one of three isomers of nitroaniline. It is an intermediate in the production of dyes, antioxidants, pharmaceuticals, gasoline, gum inhibitors, poultry medicines, and as a corrosion inhibitor.
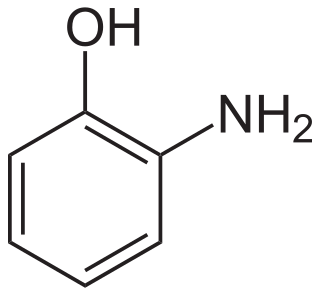
2-Aminophenol is an organic compound with the formula C6H7NO. Along with its isomer 4-aminophenol, it is an amphoteric molecule and a reducing agent. It is a useful reagent for the synthesis of dyes and heterocyclic compounds. Reflecting its slight hydrophilic character, white powder is moderately soluble in alcohols and can be recrystallized from hot water.

3-Phenylazoacetylacetone is a chemical compound used as an intermediate in the preparation of biologically active compounds, chelating agents, and dyes.

Perchloromethyl mercaptan is the organosulfur compound with the formula CCl3SCl. It is mainly used as an intermediate for the synthesis of dyes and fungicides (captan, folpet). It is a colorless oil, although commercial samples are yellowish. It is insoluble in water but soluble in organic solvents. It has a foul, unbearable, acrid odor. Perchloromethyl mercaptan is the original name. The systematic name is trichloromethanesulfenyl chloride, because the compound is a sulfenyl chloride, not a mercaptan.
3-Nitrotoluene or meta-nitrotoluene is an organic compound with the formula CH3C6H4NO2. It is one of three isomers of nitrotoluene. A yellow liquid, it is used in the manufacture of meta-toluidine, which is an intermediate in the production of various dyes.
2-Nitrotoluene or ortho-nitrotoluene is an organic compound with the formula CH3C6H4NO2. It is pale yellow liquid that crystallizes in two forms, called α (−9.27 °C) and β (−3.17 °C). It is mainly a precursor to o-toluidine, which is an intermediate in the production of various dyes.
In chemistry, the term chromogen refers to a colourless chemical compound that can be converted by chemical reaction into a compound which can be described as "coloured". There is no universally agreed definition of the term. Various dictionaries give the following definitions:
Plastic colorants are chemical compounds used to color plastic. Those compounds come in a form of dyes and pigments. The type of a colorant is chosen based on the type of a polymeric resin, that needs to be colored. Dyes are usually used with polycarbonates, polystyrene and acrylic polymers. Pigments are better suited for use with polyolefins.

4-Aminoacetanilide or paracetamin is a chemical compound which is a amino derivative of acetanilide and para-isomer of aminoacetanilide. There are two other isomers of aminoacetanilide, 2-aminoacetanilide and 3-aminoacetanilide. Aminoacetanilide derivatives are important synthetic intermediates in heterocyclic and aromatic synthesis. These derivatives have found applications in pharmaceutical industry and dyes and pigment industry.
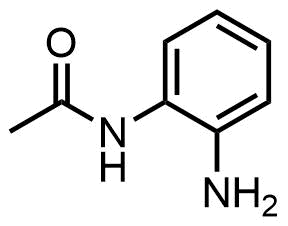
2-Aminoacetanilide is a chemical compound which is a amino derivative of acetanilide and ortho-isomer of aminoacetanilide. There are two other isomers of aminoacetanilide, 3-aminoacetanilide and 4-aminoacetanilide. Aminoacetanilide derivatives are important synthetic intermediates in heterocyclic and aromatic synthesis. These derivatives have found applications in pharmaceutical industry and dyes and pigment industry.

3'-Aminoacetanilide is a chemical compound which is a amino derivative of acetanilide and meta-isomer of aminoacetanilide. There are two other isomers of aminoacetanilide, 2-aminoacetanilide and 4-aminoacetanilide. Aminoacetanilide derivatives are important synthetic intermediates in heterocyclic and aromatic synthesis. These derivatives have found applications in pharmaceutical industry and dyes and pigment industry.
















