Related Research Articles
In chemistry, a racemic mixture or racemate, is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates.

In chemistry, an enantiomer – also called optical isomer, antipode, or optical antipode – is one of two stereoisomers that are non-superposable onto their own mirror image. Enantiomers are much like one's right and left hands, when looking at the same face, they cannot be superposed onto each other. No amount of reorientation in three spatial dimensions will allow the four unique groups on the chiral carbon to line up exactly. The number of stereoisomers a molecule has can be determined by the number of chiral carbons it has. Stereoisomers include both enantiomers and diastereomers.

A meso compound or meso isomer is an optically inactive isomer in a set of stereoisomers, at least two of which are optically active. This means that despite containing two or more stereocenters, the molecule is not chiral. A meso compound is "superimposable" on its mirror image. Two objects can be superimposed if all aspects of the objects coincide and it does not produce a "(+)" or "(-)" reading when analyzed with a polarimeter. The name is derived from the Greek mésos meaning “middle”.

In chemistry, a molecule or ion is called chiral if it cannot be superposed on its mirror image by any combination of rotations, translations, and some conformational changes. This geometric property is called chirality. The terms are derived from Ancient Greek χείρ (cheir) 'hand'; which is the canonical example of an object with this property.

Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric products in unequal amounts."
In stereochemistry, enantiomeric excess (ee) is a measurement of purity used for chiral substances. It reflects the degree to which a sample contains one enantiomer in greater amounts than the other. A racemic mixture has an ee of 0%, while a single completely pure enantiomer has an ee of 100%. A sample with 70% of one enantiomer and 30% of the other has an ee of 40%.

Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. This spectroscopy is based on the measurement of absorption of electromagnetic radiations in the radio frequency region from roughly 4 to 900 MHz. Absorption of radio waves in the presence of magnetic field is accompanied by a special type of nuclear transition, and for this reason, such type of spectroscopy is known as Nuclear Magnetic Resonance Spectroscopy. The sample is placed in a magnetic field and the NMR signal is produced by excitation of the nuclei sample with radio waves into nuclear magnetic resonance, which is detected with sensitive radio receivers. The intramolecular magnetic field around an atom in a molecule changes the resonance frequency, thus giving access to details of the electronic structure of a molecule and its individual functional groups. As the fields are unique or highly characteristic to individual compounds, in modern organic chemistry practice, NMR spectroscopy is the definitive method to identify monomolecular organic compounds.
Derivatization is a technique used in chemistry which converts a chemical compound into a product of similar chemical structure, called a derivative.

Proton nuclear magnetic resonance is the application of nuclear magnetic resonance in NMR spectroscopy with respect to hydrogen-1 nuclei within the molecules of a substance, in order to determine the structure of its molecules. In samples where natural hydrogen (H) is used, practically all the hydrogen consists of the isotope 1H.
A chiral derivatizing agent (CDA) also known as a chiral resolving reagent, is a chiral auxiliary used to convert a mixture of enantiomers into diastereomers in order to analyze the quantities of each enantiomer present within the mix. Analysis can be conducted by spectroscopy or by chromatography. The use of chiral derivatizing agents has declined with the popularization of chiral HPLC. Besides analysis, chiral derivatization is also used for chiral resolution, the actual physical separation of the enantiomers.
The Kiliani–Fischer synthesis, named for German chemists Heinrich Kiliani and Emil Fischer, is a method for synthesizing monosaccharides. It proceeds via synthesis and hydrolysis of a cyanohydrin, followed by reduction of the intermediate acid to the aldehyde, thus elongating the carbon chain of an aldose by one carbon atom while preserving stereochemistry on all the previously present chiral carbons. The new chiral carbon is produced with both stereochemistries, so the product of a Kiliani–Fischer synthesis is a mixture of two diastereomeric sugars, called epimers. For example, D-arabinose is converted to a mixture of D-glucose and D-mannose.
Chiral resolution, or enantiomeric resolution, is a process in stereochemistry for the separation of racemic compounds into their enantiomers. It is an important tool in the production of optically active compounds, including drugs. Another term with the same meaning is optical resolution.
A chiral shift reagent is a reagent used in analytical chemistry for determining the optical purity of a sample. Some analytical techniques such as HPLC and NMR, in their most commons forms, cannot distinguish enantiomers within a sample, but can distinguish diastereomers. Therefore, converting a mixture of enantiomers to a corresponding mixture of diastereomers can allow analysis.
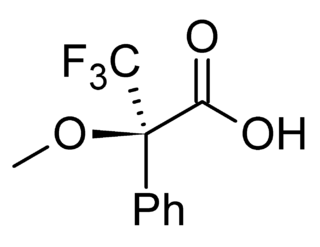
Mosher's acid, or α-methoxy-α-trifluoromethylphenylacetic acid (MTPA) is a carboxylic acid which was first used by Harry Stone Mosher as a chiral derivatizing agent. It is a chiral molecule, consisting of R and S enantiomers.

EuFOD is the chemical compound with the formula Eu(OCC(CH3)3CHCOC3F7)3, also called Eu(fod)3. This coordination compound is used primarily as a shift reagent in NMR spectroscopy. It is the premier member of the lanthanide shift reagents and was popular in the 1970s and 1980s.
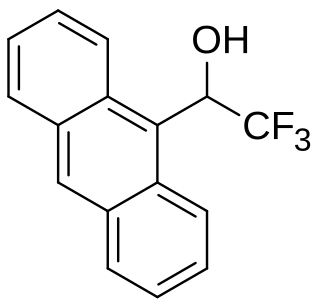
Pirkle's alcohol is an off-white, crystalline solid that is stable at room temperature when protected from light and oxygen. This chiral molecule is typically used, in nonracemic form, as a chiral shift reagent in nuclear magnetic resonance spectroscopy, in order to simultaneously determine absolute configuration and enantiomeric purity of other chiral molecules. The molecule is named after William H. Pirkle, Professor of Chemistry at the University of Illinois whose group reported its synthesis and its application as a chiral shift reagent.
An enantiopure drug is a pharmaceutical that is available in one specific enantiomeric form. Most biological molecules are present in only one of many chiral forms, so different enantiomers of a chiral drug molecule bind differently to target receptors. Chirality can be observed when the geometric properties of an object is not superimposable with its mirror image. Two forms of a molecule are formed from a chiral carbon, these two forms are called enantiomers. One enantiomer of a drug may have a desired beneficial effect while the other may cause serious and undesired side effects, or sometimes even beneficial but entirely different effects. The desired enantiomer is known as an eutomer while the undesired enantiomer is known as the distomer. When equal amounts of both enantiomers are found in a mixture, the mixture is known as a racemic mixture. If a mixture for a drug does not have a 1:1 ratio of its enantiomers it is a candidate for an enantiopure drug. Advances in industrial chemical processes have made it economical for pharmaceutical manufacturers to take drugs that were originally marketed as a racemic mixture and market the individual enantiomers, either by specifically manufacturing the desired enantiomer or by resolving a racemic mixture. On a case-by-case basis, the U.S. Food and Drug Administration (FDA) has allowed single enantiomers of certain drugs to be marketed under a different name than the racemic mixture. Also case-by-case, the United States Patent Office has granted patents for single enantiomers of certain drugs. The regulatory review for marketing approval and for patenting is independent, and differs country by country.
Ultraviolet–visible spectroscopy (UV–vis) can distinguish between enantiomers by showing a distinct Cotton effect for each isomer. UV–vis spectroscopy sees only chromophores, so other molecules must be prepared for analysis by chemical addition of a chromophore such as anthracene. Two methods are reported: the octant rule and the exciton chirality method.
Chemical compounds that come as mirror-image pairs are referred to by chemists as chiral or handed molecules. Each twin is called an enantiomer. Drugs that exhibit handedness are referred to as chiral drugs. Chiral drugs that are equimolar (1:1) mixture of enantiomers are called racemic drugs and these are obviously devoid of optical rotation. The most commonly encountered stereogenic unit, that confers chirality to drug molecules are stereogenic center. Stereogenic center can be due to the presence of tetrahedral tetra coordinate atoms (C,N,P) and pyramidal tricoordinate atoms (N,S). The word chiral describes the three-dimensional architecture of the molecule and does not reveal the stereochemical composition. Hence "chiral drug" does not say whether the drug is racemic, single enantiomer or some other combination of stereoisomers. To resolve this issue Joseph Gal introduced a new term called unichiral. Unichiral indicates that the stereochemical composition of a chiral drug is homogenous consisting of a single enantiomer.
Chiral analysis refers to the quantification of component enantiomers of racemic drug substances or pharmaceutical compounds. Other synonyms commonly used include enantiomer analysis, enantiomeric analysis, and enantioselective analysis. Chiral analysis includes all analytical procedures focused on the characterization of the properties of chiral drugs. Chiral analysis is usually performed with chiral separation methods where the enantiomers are separated on an analytical scale and simultaneously assayed for each enantiomer.
References
- ↑ David Parker. "NMR determination of enantiomeric purity." Chem. Rev. 1991, 91, 1441–1457.
- ↑ Frank J. Hollis. "NMR Through the Looking Glass: Uses of NMR Spectroscopy in the Analysis and Synthesis of Chiral Pharmaceuticals." 1994.