Related Research Articles
The Precambrian is the earliest part of Earth's history, set before the current Phanerozoic Eon. The Precambrian is so named because it preceded the Cambrian, the first period of the Phanerozoic Eon, which is named after Cambria, the Latinized name for Wales, where rocks from this age were first studied. The Precambrian accounts for 88% of the Earth's geologic time.

Paleoclimatology is the scientific study of climates predating the invention of meteorological instruments, when no direct measurement data were available. As instrumental records only span a tiny part of Earth's history, the reconstruction of ancient climate is important to understand natural variation and the evolution of the current climate.

Banded iron formations are distinctive units of sedimentary rock consisting of alternating layers of iron oxides and iron-poor chert. They can be up to several hundred meters in thickness and extend laterally for several hundred kilometers. Almost all of these formations are of Precambrian age and are thought to record the oxygenation of the Earth's oceans. Some of the Earth's oldest rock formations, which formed about 3,700 million years ago (Ma), are associated with banded iron formations.

The Proterozoic is the third of the four geologic eons of Earth's history, spanning the time interval from 2500 to 538.8 Mya, the longest eon of the Earth's geologic time scale. It is preceded by the Archean and followed by the Phanerozoic, and is the most recent part of the Precambrian "supereon".

The Archean Eon, in older sources sometimes called the Archaeozoic, is the second of the four geologic eons of Earth's history, preceded by the Hadean Eon and followed by the Proterozoic. The Archean represents the time period from 4,031 to 2,500 Mya. The Late Heavy Bombardment is hypothesized to overlap with the beginning of the Archean. The Huronian glaciation occurred at the end of the eon.

The atmosphere of Earth is composed of a layer of gas mixture that surrounds the Earth's planetary surface, known collectively as air, with variable quantities of suspended aerosols and particulates, all retained by Earth's gravity. The atmosphere serves as a protective buffer between the Earth's surface and outer space, shields the surface from most meteoroids and ultraviolet solar radiation, keeps it warm and reduces diurnal temperature variation through heat retention, redistributes heat and moisture among different regions via air currents, and provides the chemical and climate conditions allowing life to exist and evolve on Earth.

An atmosphere is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosphere is the outer region of a star, which includes the layers above the opaque photosphere; stars of low temperature might have outer atmospheres containing compound molecules.
A reducing atmosphere is an atmospheric condition in which oxidation is prevented by absence of oxygen and other oxidizing gases or vapours, and which may contain actively reductant gases such as hydrogen, carbon monoxide, methane and hydrogen sulfide that would be readily oxidized to remove any free oxygen. Although Early Earth had had a reducing prebiotic atmosphere prior to the Proterozoic eon, starting at about 2.5 billion years ago in the late Neoarchaean period, the Earth's atmosphere experienced a significant rise in oxygen and transitioned to an oxidizing atmosphere with a surplus of molecular oxygen (dioxygen, O2) as the primary oxidizing agent.

Oxygen cycle refers to the movement of oxygen through the atmosphere (air), biosphere (plants and animals) and the lithosphere (the Earth’s crust). The oxygen cycle demonstrates how free oxygen is made available in each of these regions, as well as how it is used. The oxygen cycle is the biogeochemical cycle of oxygen atoms between different oxidation states in ions, oxides, and molecules through redox reactions within and between the spheres/reservoirs of the planet Earth. The word oxygen in the literature typically refers to the most common oxygen allotrope, elemental/diatomic oxygen (O2), as it is a common product or reactant of many biogeochemical redox reactions within the cycle. Processes within the oxygen cycle are considered to be biological or geological and are evaluated as either a source (O2 production) or sink (O2 consumption).

The Mesoarchean is a geologic era in the Archean Eon, spanning 3,200 to 2,800 million years ago, which contains the first evidence of modern-style plate subduction and expansion of microbial life. The era is defined chronometrically and is not referenced to a specific level in a rock section on Earth.

The important sulfur cycle is a biogeochemical cycle in which the sulfur moves between rocks, waterways and living systems. It is important in geology as it affects many minerals and in life because sulfur is an essential element (CHNOPS), being a constituent of many proteins and cofactors, and sulfur compounds can be used as oxidants or reductants in microbial respiration. The global sulfur cycle involves the transformations of sulfur species through different oxidation states, which play an important role in both geological and biological processes. Steps of the sulfur cycle are:

The Great Oxidation Event (GOE) or Great Oxygenation Event, also called the Oxygen Catastrophe, Oxygen Revolution, Oxygen Crisis or Oxygen Holocaust, was a time interval during the Earth's Paleoproterozoic era when the Earth's atmosphere and shallow seas first experienced a rise in the concentration of free oxygen. This began approximately 2.460–2.426 Ga (billion years) ago during the Siderian period and ended approximately 2.060 Ga ago during the Rhyacian. Geological, isotopic and chemical evidence suggests that biologically produced molecular oxygen (dioxygen or O2) started to accumulate in the Archean prebiotic atmosphere due to microbial photosynthesis, and eventually changed it from a weakly reducing atmosphere practically devoid of oxygen into an oxidizing one containing abundant free oxygen, with oxygen levels being as high as 10% of modern atmospheric level by the end of the GOE.
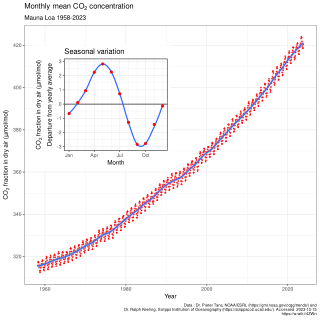
In Earth's atmosphere, carbon dioxide is a trace gas that plays an integral part in the greenhouse effect, carbon cycle, photosynthesis and oceanic carbon cycle. It is one of three main greenhouse gases in the atmosphere of Earth. The concentration of carbon dioxide in the atmosphere reached 427 ppm (0.04%) in 2024. This is an increase of 50% since the start of the Industrial Revolution, up from 280 ppm during the 10,000 years prior to the mid-18th century. The increase is due to human activity.

The Azolla event is a paleoclimatology scenario hypothesized to have occurred in the middle Eocene epoch, around 49 million years ago, when blooms of the carbon-fixing freshwater fern Azolla are thought to have happened in the Arctic Ocean. As the fern died and sank to the stagnant sea floor, they were incorporated into the sediment over a period of about 800,000 years; the resulting draw-down of carbon dioxide has been speculated to have helped reverse the planet from the "greenhouse Earth" state of the Paleocene-Eocene Thermal Maximum, when the planet was hot enough for turtles and palm trees to prosper at the poles, to the current icehouse Earth known as the Late Cenozoic Ice Age.

The carbonate–silicate geochemical cycle, also known as the inorganic carbon cycle, describes the long-term transformation of silicate rocks to carbonate rocks by weathering and sedimentation, and the transformation of carbonate rocks back into silicate rocks by metamorphism and volcanism. Carbon dioxide is removed from the atmosphere during burial of weathered minerals and returned to the atmosphere through volcanism. On million-year time scales, the carbonate-silicate cycle is a key factor in controlling Earth's climate because it regulates carbon dioxide levels and therefore global temperature.
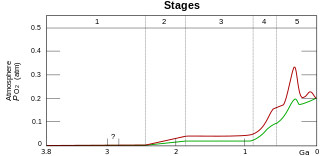
Although oxygen is the most abundant element in Earth's crust, due to its high reactivity it mostly exists in compound (oxide) forms such as water, carbon dioxide, iron oxides and silicates. Before photosynthesis evolved, Earth's atmosphere had no free diatomic elemental oxygen (O2). Small quantities of oxygen were released by geological and biological processes, but did not build up in the reducing atmosphere due to reactions with then-abundant reducing gases such as atmospheric methane and hydrogen sulfide and surface reductants such as ferrous iron.
The Boring Billion, otherwise known as the Mid Proterozoic and Earth's Middle Ages, is an informal geological time period between 1.8 and 0.8 billion years ago (Ga) during the middle Proterozoic eon spanning from the Statherian to the Tonian periods, characterized by more or less tectonic stability, climatic stasis and slow biological evolution. Although it is bordered by two different oxygenation events and two global glacial events, the Boring Billion period itself actually had very low oxygen levels and no geological evidence of glaciations.
David C. Catling is a Professor in Earth and Space Sciences at the University of Washington. He is a planetary scientist and astrobiologist whose research focuses on understanding the differences between the evolution of planets, their atmospheres, and their potential for life. He has participated in NASA's Mars exploration program and contributed research to help find life elsewhere in the solar system and on planets orbiting other stars. He is also known for his work on the evolution of Earth's atmosphere and biosphere, including how Earth's atmosphere became rich in oxygen, allowing complex life to evolve, and conditions conducive to the origin of life.

The prebiotic atmosphere is the second atmosphere present on Earth before today's biotic, oxygen-rich third atmosphere, and after the first atmosphere of Earth's formation. The formation of the Earth, roughly 4.5 billion years ago, involved multiple collisions and coalescence of planetary embryos. This was followed by a <100 million year period on Earth where a magma ocean was present, the atmosphere was mainly steam, and surface temperatures reached up to 8,000 K (14,000 °F). Earth's surface then cooled and the atmosphere stabilized, establishing the prebiotic atmosphere. The environmental conditions during this time period were quite different from today: the Sun was ~30% dimmer overall yet brighter at ultraviolet and x-ray wavelengths, there was a liquid ocean, it is unknown if there were continents but oceanic islands were likely, Earth's interior chemistry was different, and there was a larger flux of impactors hitting Earth's surface.
The Neoproterozoic Oxygenation Event (NOE), also called the Second Great Oxidation Event, was a geologic time interval between around 850 and 540 million years ago during the Neoproterozoic era, which saw a very significant increase in oxygen levels in Earth's atmosphere and oceans. Taking place after the end to the Boring Billion, an euxinic period of extremely low atmospheric oxygen spanning from the Statherian period of the Paleoproterozoic era to the Tonian period of the Neoproterozoic era, the NOE was the second major increase in atmospheric and oceanic oxygen concentration on Earth, though it was not as prominent as the Great Oxidation Event (GOE) of the Neoarchean-Paleoproterozoic boundary. Unlike the GOE, it is unclear whether the NOE was a synchronous, global event or a series of asynchronous, regional oxygenation intervals with unrelated causes.
References
- ↑ Berner, Robert A. (1998). "The carbon cycle and CO
2 over Phanerozoic time: the role of land plants". Philosophical Transactions of the Royal Society. 353 (1365): 75–82. doi:10.1098/Rstb.1998.0192. PMC 1692179 . - ↑ Berner, Robert A. (1997). "The rise of plants: their effect on weathering and atmospheric CO
2". Science. 276: 544–546. doi:10.1126/Science.276.5312.544. S2CID 128649732. - ↑ Beerling, David J.; Berner, Robert A. (2005). "Feedbacks and the coevolution of plants and atmospheric CO
2". Proceedings of the National Academy of Sciences. 102 (5). USA: 1302–1305. Bibcode:2005PNAS..102.1302B. doi: 10.1073/Pnas.0408724102 . PMC 547859 . PMID 15668402. - ↑ Som, Sanjoy M.; Catling, David C.; Harnmeijer, Jelte P.; Polivka, Peter M.; Buick, Roger (2012). "Air density 2.7 billion years ago limited to less than twice modern levels by fossil raindrop imprints". Nature. 484.7394 (7394): 359–362. Bibcode:2012Natur.484..359S. doi:10.1038/nature10890. PMID 22456703. S2CID 4410348.
- ↑ Som, Sanjoy M.; Buick, Roger; Hagadorn, James W.; Blake, Tim S.; Perreault, John M.; Harnmeijer, Jelte P.; Catling, David C. (2016). "Earth's air pressure 2.7 billion years ago constrained to less than half of modern levels". Nature Geoscience. 9 (6): 448–451. Bibcode:2016NatGe...9..448S. doi:10.1038/ngeo2713.
- ↑ Blamey, Nigel J. F.; Brand, Uwe; Parnell, John; Spear, Natalie; Lécuyer, Christophe; Benison, Kathleen; Meng, Fanwei; Ni, Pei (2016). "Paradigm shift in determining Neoproterozoic atmospheric oxygen". Geology. 44 (8): 651. Bibcode:2016Geo....44..651B. doi: 10.1130/G37937.1 . hdl: 2164/6234 .